Associations Between Asthma Diagnosis/Asthma Exacerbation and Previous Proton-Pump Inhibitor use: A Nested Case-Control Study Using a National Health Screening Cohort
- PMID: 35847037
- PMCID: PMC9279665
- DOI: 10.3389/fphar.2022.888610
Associations Between Asthma Diagnosis/Asthma Exacerbation and Previous Proton-Pump Inhibitor use: A Nested Case-Control Study Using a National Health Screening Cohort
Abstract
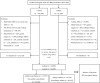
Background: Proton-pump inhibitors (PPIs) block acid secretion from gastric parietal cells; however, recent studies have reported that PPIs have antioxidant and anti-inflammatory properties in various cells. Newer PPIs are stronger inhibitors of acid secretion; however, the anti-inflammatory effects of these drugs have not been assessed. We evaluated anti-inflammatory effect of PPIs on the development of asthma/asthma exacerbation (AE) in a national health screening cohort. Methods: This case-control study comprised 64,809 participants with asthma who were 1:1 matched with controls from the Korean National Health Insurance Service-Health Screening Cohort. Conditional logistic regression analysis was used to evaluate the effect of previous PPI use on an asthma diagnosis in all participants. Unconditional logistic regression was used to assess the effect of PPI use on AE in participants with asthma. These relationships were estimated in a subgroup analysis according to PPI generation. Results: Overall, PPI use increased the risk of asthma diagnosis [adjusted odds ratio (aOR) = 1.29, 95% confidence interval (CI) = 1.23-1.35, p < 0.001]. Use of the first-generation PPIs was associated with asthma (aOR = 1.34, 95% CI = 1.18-1.52, p < 0.001), while use of second-generation PPIs was not (aOR = 0.97, 95% CI = 0.82-1.15, p = 0.748). In contrast, overall PPI use decreased the risk of AE in participants with asthma (aOR = 0.79, 95% CI = 0.75-0.84, p < 0.001), although this effect was observed only for second-generation PPIs (aOR = 0.76, 95% CI = 0.65-0.89, p = 0.001). Conclusion: PPI use increased the risk for subsequent asthma diagnosis. However, this effect was confined to first-generation PPIs. Second-generation PPIs decreased the risk of AE.
Keywords: asthma; cohort studies; exacerbation (symptom flare up); inflammation; proton pump inhibitor (PPI).
Copyright © 2022 Choi, Min, Yoo, Tan, Kim, Kim, Park, Park, Hwang, Jang and Jung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Associations between proton pump inhibitors and Alzheimer's disease: a nested case-control study using a Korean nationwide health screening cohort.Alzheimers Res Ther. 2022 Jul 1;14(1):91. doi: 10.1186/s13195-022-01032-5. Alzheimers Res Ther. 2022. PMID: 35773740 Free PMC article.
-
Comparative analysis of the risk of osteoporotic fractures with proton pump inhibitor use and histamine-2 receptor antagonist therapy in elderly women: A nationwide population-based nested case-control study.Bone. 2020 Jun;135:115306. doi: 10.1016/j.bone.2020.115306. Epub 2020 Feb 29. Bone. 2020. PMID: 32126312
-
Association between Proton Pump Inhibitor Use and Parkinson's Disease in a Korean Population.Pharmaceuticals (Basel). 2022 Mar 9;15(3):327. doi: 10.3390/ph15030327. Pharmaceuticals (Basel). 2022. PMID: 35337125 Free PMC article.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.Health Technol Assess. 2007 Dec;11(51):iii-iv, 1-164. doi: 10.3310/hta11510. Health Technol Assess. 2007. PMID: 18021578 Review.
-
Proton pump inhibitors and the risk of colorectal cancer: a systematic review and meta-analysis of observational studies.Int J Colorectal Dis. 2020 Dec;35(12):2157-2169. doi: 10.1007/s00384-020-03717-5. Epub 2020 Aug 18. Int J Colorectal Dis. 2020. PMID: 32808072
Cited by
-
Risk Factors for Acute Asthma Exacerbations in Adults With Mild Asthma.J Allergy Clin Immunol Pract. 2024 Oct;12(10):2705-2716.e6. doi: 10.1016/j.jaip.2024.05.034. Epub 2024 May 29. J Allergy Clin Immunol Pract. 2024. PMID: 38821437
References
-
- American Lung Association Asthma Clinical Research Centers Mastronarde J. G., Anthonisen N. R., Castro M., Holbrook J. T., Leone F. T., Teague W. G., et al. (2009). Efficacy of Esomeprazole for Treatment of Poorly Controlled Asthma. N. Engl. J. Med. 360 (15), 1487–1499. 10.1056/NEJMoa0806290 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

