Environmental Factors Associated With Soil Prevalence of the Melioidosis Pathogen Burkholderia pseudomallei: A Longitudinal Seasonal Study From South West India
- PMID: 35847064
- PMCID: PMC9283100
- DOI: 10.3389/fmicb.2022.902996
Environmental Factors Associated With Soil Prevalence of the Melioidosis Pathogen Burkholderia pseudomallei: A Longitudinal Seasonal Study From South West India
Abstract
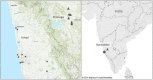
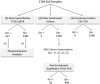
Melioidosis is a seasonal infectious disease in tropical and subtropical areas caused by the soil bacterium Burkholderia pseudomallei. In many parts of the world, including South West India, most cases of human infections are reported during times of heavy rainfall, but the underlying causes of this phenomenon are not fully understood. India is among the countries with the highest predicted melioidosis burden globally, but there is very little information on the environmental distribution of B. pseudomallei and its determining factors. The present study aimed (i) to investigate the prevalence of B. pseudomallei in soil in South West India, (ii) determine geochemical factors associated with B. pseudomallei presence and (iii) look for potential seasonal patterns of B. pseudomallei soil abundance. Environmental samplings were performed in two regions during the monsoon and post-monsoon season and summer from July 2016 to November 2018. We applied direct quantitative real time PCR (qPCR) together with culture protocols to overcome the insufficient sensitivity of solely culture-based B. pseudomallei detection from soil. A total of 1,704 soil samples from 20 different agricultural sites were screened for the presence of B. pseudomallei. Direct qPCR detected B. pseudomallei in all 20 sites and in 30.2% (517/1,704) of all soil samples, whereas only two samples from two sites were culture-positive. B. pseudomallei DNA-positive samples were negatively associated with the concentration of iron, manganese and nitrogen in a binomial logistic regression model. The highest number of B. pseudomallei-positive samples (42.6%, p < 0.0001) and the highest B. pseudomallei loads in positive samples [median 4.45 × 103 genome equivalents (GE)/g, p < 0.0001] were observed during the monsoon season and eventually declined to 18.9% and a median of 1.47 × 103 GE/g in summer. In conclusion, our study from South West India shows a wide environmental distribution of B. pseudomallei, but also considerable differences in the abundance between sites and within single sites. Our results support the hypothesis that nutrient-depleted habitats promote the presence of B. pseudomallei. Most importantly, the highest B. pseudomallei abundance in soil is seen during the rainy season, when melioidosis cases occur.
Keywords: Burkholderia pseudomallei; South West India; environmental surveillance; melioidosis; physicochemical factors; seasonal variation; soil.
Copyright © 2022 Shaw, Assig, Tellapragada, Wagner, Choudhary, Göhler, Eshwara, Steinmetz and Mukhopadhyay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



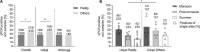



References
-
- Anuntagool N., Naigowit P., Petkanchanapong V., Aramsri P., Panichakul T., Sirisinha S. (2000). Monoclonal antibody-based rapid identification of Burkholderia pseudomallei in blood culture fluid from patients with community-acquired septicaemia. J. Med. Microbiol. 49 1075–1078. 10.1099/0022-1317-49-12-1075 - DOI - PubMed
-
- Azadi A., Baghernejad M., Gholami A., Shakeri S. (2021). Forms and distribution pattern of soil Fe (Iron) and Mn (Manganese) oxides due to long-term rice cultivation in fars Province Southern Iran. Commun. Soil Sci. Plant Analys. 52 1894–1911. 10.1080/00103624.2021.1900226 - DOI
LinkOut - more resources
Full Text Sources

