In vitro Anti-Hantavirus Activity of Protein Kinase Inhibitor 8G1 Targeting AKT/mTOR/eIF4E Signaling Pathway
- PMID: 35847100
- PMCID: PMC9279581
- DOI: 10.3389/fmicb.2022.880258
In vitro Anti-Hantavirus Activity of Protein Kinase Inhibitor 8G1 Targeting AKT/mTOR/eIF4E Signaling Pathway
Abstract
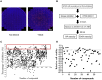
Hantaan virus (HTNV) is the main cause of hemorrhagic fever with renal syndrome (HFRS) around the world, which results in profound morbidity and mortality. However, there are currently no FDA-approved therapeutics or vaccines against HFRS. To find new anti-HTNV drugs, the inhibitory activity of 901 small molecule kinase inhibitors against HTNV is analyzed. Among these compounds, compound 8G1 inhibits HTNV with a relatively high inhibition rate and lower toxicity. The viral titer and nucleocapsid protein of HTNV are reduced after compound 8G1 treatment in a dose-dependent manner at concentrations ranging from 1 to 20 μM. In addition, the administration of compound 8G1 at the early stage of HTNV infection can inhibit the replication of HTNV. The molecular docking result reveals that compound 8G1 forms interactions with the key amino acid residues of serine/threonine-protein kinase B (Akt), which is responsible for the observed affinity. Then, the mammalian target of rapamycin (mTOR) and eukaryotic translation initiation factor 4E (eIF4E) signaling pathways are inhibited. Our results may help to design novel targets for therapeutic intervention against HTNV infection and to understand the anti-HTNV mechanism of protein kinase inhibitors.
Keywords: Hantaan virus; inhibitor; nucleocapsid protein; protein kinase; the mammalian target of rapamycin.
Copyright © 2022 Li, Wang, Ying, Kong, Zhang, Dong, Liu, Zhai, Chen, Jia, Xue, Li and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Hantavirus: an overview and advancements in therapeutic approaches for infection.Front Microbiol. 2023 Oct 12;14:1233433. doi: 10.3389/fmicb.2023.1233433. eCollection 2023. Front Microbiol. 2023. PMID: 37901807 Free PMC article. Review.
-
Coumarin Derivative N6 as a Novel anti-hantavirus Infection Agent Targeting AKT.Front Pharmacol. 2021 Dec 6;12:745646. doi: 10.3389/fphar.2021.745646. eCollection 2021. Front Pharmacol. 2021. PMID: 34938178 Free PMC article.
-
An algal lectin griffithsin inhibits Hantaan virus infection in vitro and in vivo.Front Cell Infect Microbiol. 2022 Dec 12;12:881083. doi: 10.3389/fcimb.2022.881083. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36579342 Free PMC article.
-
Phosphorylation of the nucleocapsid protein of Hantaan virus by casein kinase II.J Microbiol. 2015 May;53(5):343-7. doi: 10.1007/s12275-015-5095-3. Epub 2015 May 3. J Microbiol. 2015. PMID: 25935306
-
RNA-Seq Revealed a Circular RNA-microRNA-mRNA Regulatory Network in Hantaan Virus Infection.Front Cell Infect Microbiol. 2020 Mar 13;10:97. doi: 10.3389/fcimb.2020.00097. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32232013 Free PMC article. Review.
Cited by
-
Hantavirus: an overview and advancements in therapeutic approaches for infection.Front Microbiol. 2023 Oct 12;14:1233433. doi: 10.3389/fmicb.2023.1233433. eCollection 2023. Front Microbiol. 2023. PMID: 37901807 Free PMC article. Review.
-
Puumala orthohantavirus: prevalence, biology, disease, animal models and recent advances in therapeutics development and structural biology.Front Immunol. 2025 May 8;16:1575112. doi: 10.3389/fimmu.2025.1575112. eCollection 2025. Front Immunol. 2025. PMID: 40406115 Free PMC article. Review.
References
-
- Hayashi Y., Watanabe H., Okudaira N., Otomo E., Endo S. (1981). The effects of butoctamide hydrogen succinate (bahs) on the sleep of the aged people (author's transl). No To Shinkei 33, 1243–1250. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

