Clinical Characteristics of Omicron SARS-CoV-2 Variant Infection After Non-mRNA-Based Vaccination in China
- PMID: 35847120
- PMCID: PMC9279136
- DOI: 10.3389/fmicb.2022.901826
Clinical Characteristics of Omicron SARS-CoV-2 Variant Infection After Non-mRNA-Based Vaccination in China
Abstract
Introduction: To date, little is known about the real-world protective role of Chinese inactivated and recombinant coronavirus disease 2019 (COVID-19) vaccines under the background of the long-term "Dynamic Zero COVID-19 Case" (i.e., no infection source) in China, especially when facing the widespread Omicron severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant infection.
Methods: In this prospective, single-center cohort study, the clinical characteristics of post-vaccination Omicron SARS-CoV-2 variant infection were investigated in the initial largest outbreak of Omicron SARS-CoV-2 variant infection that occurred between the 8 January, 2022 and 29 January, 2022 in Anyang City, Henan Province, China. The primary endpoints were the rates of severe and critical diseases or death. The secondary endpoints were the SARS-CoV-2 shedding duration and length of hospitalization.
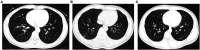
Results: A total of 380 post-vaccination patients infected with the Omicron SARS-CoV-2 variant were enrolled. The median age was 18 (interquartile range [IQR] 17-35) years, 219 (57.6%) cases were female, and 247 (65.0%) cases were students. Before confirmation of Omicron SARS-CoV-2 variant infection, patients had 3 (IQR 2-4) days of dry cough (40.3%), nasal congestion (26.3%), and sore throat (26.3%). On admission, 294 (77.4%) cases had normal chest computerized tomography (CT) imaging. Additionally, only 5 (1.3%), 30 (7.9%), 4 (4/342, 1.2%), and 7 (7/379, 0.2%) patients had lymphocyte counts <800 per mm3, C-reactive protein levels >10 mg/L, lactate dehydrogenase levels ≥250 U/L, and D-dimer levels ≥0.5 mg/L on admission, respectively. During hospitalization, 308 (81.1%) and 72 (18.9%) were identified as mild and moderate cases, respectively, and no one progressed to severe and critical types, with a SARS-CoV-2 shedding period and length of hospital stay of 17 (IQR 12-22) and 19 (IQR 15-24) days, respectively.
Conclusion: The current study found that approximately 80% of individuals infected with the Omicron SARS-CoV-2 variant were mild, approximately 20% of patients were moderate, and no severe, critical, or fatal cases were identified in a prospective cohort including 380 participants vaccinated with non-mRNA-based vaccines.
Discussion: This study supports the consideration of policy adjustments and changes to prevent and control the Omicron-predominant COVID-19 in China and other regions with high SARS-CoV-2 vaccination rates.
Keywords: China; coronavirus disease 2019; omicron variant; severe acute respiratory syndrome coronavirus 2; vaccination; viral shedding.
Copyright © 2022 Zeng, Lv, Liu, Jiang, Huang, Li and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

