Acute Unilateral Peripheral Vestibulopathy After COVID-19 Vaccination: Initial Experience in a Tertiary Neurotology Center
- PMID: 35847228
- PMCID: PMC9283640
- DOI: 10.3389/fneur.2022.917845
Acute Unilateral Peripheral Vestibulopathy After COVID-19 Vaccination: Initial Experience in a Tertiary Neurotology Center
Abstract
Objective: The aim of the present study was to identify patients who developed acute unilateral peripheral vestibulopathy (AUPVP) after COVID-19 vaccination.
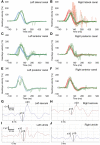
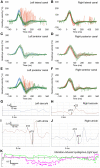
Methods: For this single-center, retrospective study, we screened the medical records of our tertiary interdisciplinary neurotology center for patients who had presented with AUPVP within 30 days after COVID-19 vaccination (study period: 1 June-31 December 2021). The initial diagnosis of AUPVP was based on a comprehensive bedside neurotological examination. Laboratory vestibular testing (video head impulse test, cervical and ocular vestibular evoked myogenic potentials, dynamic visual acuity, subjective visual vertical, video-oculography, caloric testing) was performed 1-5 months later.
Results: Twenty-six patients were diagnosed with AUPVP within the study period. Of those, n = 8 (31%) had developed acute vestibular symptoms within 30 days after COVID-19 vaccination (mean interval: 11.9 days, SD: 4.8, range: 6-20) and were thus included in the study. The mean age of the patients (two females, six males) was 46 years (SD: 11.7). Seven patients had received the Moderna mRNA vaccine and one the Pfizer/BioNTech mRNA vaccine. All patients displayed a horizontal(-torsional) spontaneous nystagmus toward the unaffected ear and a pathological clinical head impulse test toward the affected ear on initial clinical examination. Receptor-specific laboratory vestibular testing performed 1-5 months later revealed recovery of vestibular function in two patients, and heterogeneous lesion patterns of vestibular endorgans in the remaining six patients.
Discussion and conclusions: The present study should raise clinicians' awareness for AUPVP after COVID-19 vaccination. The relatively high fraction of such cases among our AUPVP patients may be due to a certain selection bias at a tertiary neurotology center. Patients presenting with acute vestibular symptoms should be questioned about their vaccination status and the date of the last vaccination dose. Furthermore, cases of AUPVP occurring shortly after a COVID-19 vaccination should be reported to the health authorities to help determining a possible causal relationship.
Keywords: COVID-19; SARS-CoV-2; acute unilateral peripheral vestibulopathy; autoimmune cross-reactivity; herpes simplex virus; vaccination; vestibular neuritis.
Copyright © 2022 Schmid, Bächinger, Pangalu, Straumann and Dlugaiczyk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer SH declared a shared parent affiliation with the authors to the handling editor at the time of review.
Figures
References
-
- John Hopkins University Corona Ressource Center . Available online at: https://coronavirus.jhu.edu/ (accessed April 2, 2022).
-
- Centers for Disease Control and Prevention . Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html... (accessed April 2, 2022).
LinkOut - more resources
Full Text Sources
Miscellaneous

