A Two-Step Magnetron Sputtering Approach for the Synthesis of Cu2ZnSnS4 Films from Cu2SnS3\ZnS Stacks
- PMID: 35847258
- PMCID: PMC9281322
- DOI: 10.1021/acsomega.2c02475
A Two-Step Magnetron Sputtering Approach for the Synthesis of Cu2ZnSnS4 Films from Cu2SnS3\ZnS Stacks
Abstract
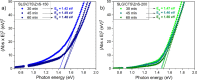
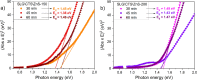
Cu2ZnSnS4 (CZTS) is regarded as one of the emerging materials for next-generation thin film solar cells. However, its synthesis is complex, and obtaining a single-phase CZTS thin film is difficult. This work reports the elaboration of Cu2ZnSnS4 thin films by a sequential magnetron sputtering deposition of Cu2SnS3 (CTS) and ZnS as stacked films. Initially, the CTS films were prepared on a soda lime glass substrate by annealing Cu and SnS2 stacked layers. Second, ZnS was deposited by magnetron sputtering on the CTS films. The CTS\ZnS stacks were then annealed in Sn + S or S atmospheres. The tetragonal CZTS structure was obtained and confirmed by grazing incidence X-ray diffraction and Raman spectroscopy. The morphological and compositional characteristics, measured by scanning electron microscopy and energy-dispersive spectroscopy, revealed large grains and dense surfaces with the elemental composition close to the intended stoichiometry. Additional X-ray photoemission spectroscopy measurements were performed to determine the surface chemistry and particularities of the obtained films. The optical properties, determined using conventional spectroscopy, showed optimal absorber layer band gap values ranging between 1.38 and 1.50 eV. The electrical measurements showed that all the films are p-type with high carrier concentrations in the range of 1015 to 1020 cm-3. This new synthesis route for CZTS opens the way to obtain high-quality films by an industry-compatible method.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Wang Z.; Demopoulos G. P. Nanoscale Photo-Absorbing Kesterite Grown on Anatase Mesoscopic Films by Sequential Binary Chalcogenide Solution Deposition-Exchange, Annealing, and Etching. Cryst. Growth Des. 2016, 16, 3618–3630. 10.1021/acs.cgd.6b00033. - DOI
-
- Yu K.; Carter E. A. A Strategy to Stabilize Kesterite CZTS for High-Performance Solar Cells. Chem. Mater. 2015, 27, 2920–2927. 10.1021/acs.chemmater.5b00172. - DOI
-
- Azmi S.; Moujib A.; Layachi O. A.; Matei E.; Galca A. C.; Zaki M. Y.; Secu M.; Rusu M. I.; Grigorescu C. E. A.; Khoumri E. M. Towards Phase Pure Kesterite Cu2ZnSnS4 Absorber Layers Growth via Single Step Free Sulfurization Electrodeposition under a Fix Applied Potential on Mo Substrate. J. Alloys Compd. 2020, 842, 15582110.1016/j.jallcom.2020.155821. - DOI
-
- El Khouja O.; Galca A. C.; Zaki M. Y.; Talbi A.; Ahmoum H.; Nouneh K.; Ebn Touhami M.; Taibi M.; Matei E.; Enculescu M.; Pintilie L. Secondary Phases and Their Influence on Optical and Electrical Properties of Electrodeposited Cu2FeSnS4 Films. Appl. Phys. A: Mater. Sci. Process. 2021, 127, 887.10.1007/s00339-021-05038-y. - DOI
-
- Chesman A. S. R.; van Embden J.; Duffy N. W.; Webster N. A. S.; Jasieniak J. J. In Situ Formation of Reactive Sulfide Precursors in the One-Pot, Multigram Synthesis of Cu2ZnSnS4 Nanocrystals. Cryst. Growth Des. 2013, 13, 1712–1720. 10.1021/cg4000268. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

