A 3D bioprinted nano-laponite hydrogel construct promotes osteogenesis by activating PI3K/AKT signaling pathway
- PMID: 35847377
- PMCID: PMC9278073
- DOI: 10.1016/j.mtbio.2022.100342
A 3D bioprinted nano-laponite hydrogel construct promotes osteogenesis by activating PI3K/AKT signaling pathway
Abstract
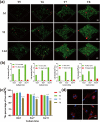
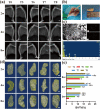
Development of nano-laponite as bioinks based on cell-loaded hydrogels has recently attracted significant attention for promoting bone defect repairs and regeneration. However, the underlying mechanisms of the positive function of laponite in hydrogel was not fully explored. In this study, the effect of 3D bioprinted nano-laponite hydrogel construct on bone regeneration and the potential mechanism was explored in vitro and in vivo. In vitro analyses showed that the 3D construct protected encapsulated cells from shear stresses during bioprinting, promoted cell growth and cell spreading, and BMSCs at a density of 107/mL exhibited an optimal osteogenesis potential. Osteogenic differentiation and ectopic bone formation of BMSCs encapsulated inside the 3D construct were explored by determination of calcium deposition and x-ray, micro-CT analysis, respectively. RNA sequencing revealed that activation of PI3K/AKT signaling pathway of BMSCs inside the laponite hydrogel significantly upregulated expression of osteogenic related proteins. Expression of osteogenic proteins was significantly downregulated when the PI3K/AKT pathway was inhibited. The 3D bioprinted nano-laponite hydrogel construct exhibited a superior ability for bone regeneration in rat bones with defects compared with groups without laponite as shown by micro-CT and histological examination, while the osteogenesis activity was weakened by applications of a PI3K inhibitor. In summary, the 3D bioprinted nano-laponite hydrogel construct promoted bone osteogenesis by promoting cell proliferation, differentiation through activation of the PI3K/AKT signaling pathway.
Keywords: Bioprinting; Cell density; Nano-laponite; Osteogenesis; Signal pathway.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Functionalized 3D-printed GelMA/Laponite hydrogel scaffold promotes BMSCs recruitment through osteoimmunomodulatory enhance osteogenic via AMPK/mTOR signaling pathway.Mater Today Bio. 2024 Sep 23;29:101261. doi: 10.1016/j.mtbio.2024.101261. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39381262 Free PMC article.
-
3D bioprinting of in situ vascularized tissue engineered bone for repairing large segmental bone defects.Mater Today Bio. 2022 Aug 8;16:100382. doi: 10.1016/j.mtbio.2022.100382. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36033373 Free PMC article.
-
3D-bioprinted functional scaffold based on synergistic induction of i-PRF and laponite exerts efficient and personalized bone regeneration via miRNA-mediated TGF-β/Smads signaling.Int J Surg. 2025 May 1;111(5):3193-3211. doi: 10.1097/JS9.0000000000002312. Int J Surg. 2025. PMID: 40035712 Free PMC article.
-
3D printing of reduced glutathione grafted gelatine methacrylate hydrogel scaffold promotes diabetic bone regeneration by activating PI3K/Akt signaling pathway.Int J Biol Macromol. 2022 Dec 1;222(Pt A):1175-1191. doi: 10.1016/j.ijbiomac.2022.09.236. Epub 2022 Sep 28. Int J Biol Macromol. 2022. PMID: 36181886
-
3D-Bioprinted Osteoblast-Laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis both In Vitro and In Vivo.Adv Sci (Weinh). 2017 Nov 24;5(3):1700550. doi: 10.1002/advs.201700550. eCollection 2018 Mar. Adv Sci (Weinh). 2017. PMID: 29593958 Free PMC article.
Cited by
-
Functionalized 3D-printed GelMA/Laponite hydrogel scaffold promotes BMSCs recruitment through osteoimmunomodulatory enhance osteogenic via AMPK/mTOR signaling pathway.Mater Today Bio. 2024 Sep 23;29:101261. doi: 10.1016/j.mtbio.2024.101261. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39381262 Free PMC article.
-
Periodic static compression of micro-strain pattern regulates endochondral bone formation.Front Bioeng Biotechnol. 2024 Mar 27;12:1356135. doi: 10.3389/fbioe.2024.1356135. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38600948 Free PMC article.
-
Combinatory Nanovesicle with siRNA-Loaded Extracellular Vesicle and IGF-1 for Osteoarthritis Treatments.Int J Mol Sci. 2024 May 11;25(10):5242. doi: 10.3390/ijms25105242. Int J Mol Sci. 2024. PMID: 38791285 Free PMC article.
-
Multiplexed PLGA scaffolds with nitric oxide-releasing zinc oxide and melatonin-modulated extracellular vesicles for severe chronic kidney disease.J Adv Res. 2025 Mar;69:75-89. doi: 10.1016/j.jare.2024.03.018. Epub 2024 Mar 25. J Adv Res. 2025. PMID: 38537702 Free PMC article.
-
Nano-laponite encapsulated coaxial fiber scaffold promotes endochondral osteogenesis.Regen Biomater. 2024 Jul 4;11:rbae080. doi: 10.1093/rb/rbae080. eCollection 2024. Regen Biomater. 2024. PMID: 39055302 Free PMC article.
References
-
- Sun W., Starly B., Daly A.C., Burdick J.A., Groll J., Skeldon G., Shu W., Sakai Y., Shinohara M., Nishikawa M., Jang J., Cho D.W., Nie M., Takeuchi S., Ostrovidov S., Khademhosseini A., Kamm R.D., Mironov V., Moroni L., Ozbolat I.T. The bioprinting roadmap. Biofabrication. 2020;12(2) doi: 10.1088/1758-5090/ab5158. - DOI - PubMed
LinkOut - more resources
Full Text Sources

