Lentiviral interferon: A novel method for gene therapy in bladder cancer
- PMID: 35847448
- PMCID: PMC9251210
- DOI: 10.1016/j.omto.2022.06.005
Lentiviral interferon: A novel method for gene therapy in bladder cancer
Abstract
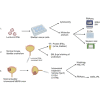
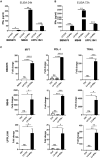
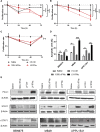
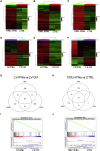
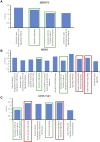
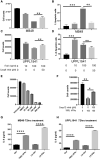
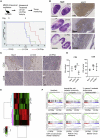
Interferon alpha (IFNα) gene therapy is emerging as a new treatment option for patients with non-muscle invasive bladder cancer (NMIBC). Adenoviral vectors expressing IFNα have shown clinical efficacy treating bacillus Calmette-Guerin (BCG)-unresponsive bladder cancer (BLCA). However, transient transgene expression and adenoviral immunogenicity may limit therapeutic activity. Lentiviral vectors can achieve stable transgene expression and are less immunogenic. In this study, we evaluated lentiviral vectors expressing murine IFNα (LV-IFNα) and demonstrate IFNα expression by transduced murine BLCA cell lines, bladder urothelium, and within the urine following intravesical instillation. Murine BLCA cell lines (MB49 and UPPL1541) were sensitive to IFN-mediated cell death after LV-IFNα, whereas BBN975 was inherently resistant. Upregulation of interleukin-6 (IL-6) predicted sensitivity to IFN-mediated cell death mediated by caspase signaling, which when inhibited abrogated IFN-mediated cell killing. Intravesical therapy with LV-IFNα/Syn3 in a syngeneic BLCA model significantly improved survival, and molecular analysis of treated tumors revealed upregulation of apoptotic and immune-cell-mediated death pathways. In particular, biomarker discovery analysis identified three clinically actionable targets, PD-L1, epidermal growth factor receptor (EGFR), and ALDHA1A, in murine tumors treated with LV-IFNα/Syn3. Our findings warrant the comparison of adenoviral and LV-IFNα and the study of novel combination strategies with IFNα gene therapy for the BLCA treatment.
Keywords: IL-6; MB49; bladder cancer; gene therapy; interferon alpha; intravesical; lentiviral vector.
© 2022 The Author(s).
Conflict of interest statement
C.P.D. received personal compensation from FKD Therapies, Oy for consulting and advisory services, including serving as the Independent Chairman of steering committee for the phase 3 nadofaragene firadenovec (rAd-IFNα/Syn3) trial.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

