Prmt1 upregulated by Hdc deficiency aggravates acute myocardial infarction via NETosis
- PMID: 35847488
- PMCID: PMC9279636
- DOI: 10.1016/j.apsb.2021.10.016
Prmt1 upregulated by Hdc deficiency aggravates acute myocardial infarction via NETosis
Abstract
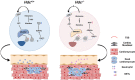
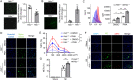
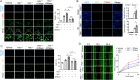
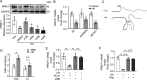
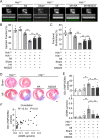
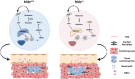
Neutrophils are mobilized and recruited to the injured heart after myocardial infarction, and neutrophil count has been clinically implicated to be associated with coronary disease severity. Histidine decarboxylase (HDC) has been implicated in regulating reactive oxidative species (ROS) and the differentiation of myeloid cells. However, the effect of HDC on neutrophils after myocardial infarction remains unclear. Here, we found that neutrophils were disorderly recruited into the ischemic injured area of the myocardium of Hdc deficiency (Hdc -/-) mice. Moreover, Hdc deficiency led to attenuated adhesion but enhanced migration and augmented ROS/neutrophil extracellular traps (NETs) production in neutrophils. Hdc -/- mouse-derived NETs promoted cardiomyocyte death and cardiac fibroblast proliferation/migration. Furthermore, protein arginine methyltransferase 1 (PRMT1) was increased in Hdc -/- mouse-derived neutrophils but decreased with exogenous histamine treatment. Its expression could be rescued by blocking histamine receptor 1 (H1R), inhibiting ATP synthesis or reducing SWItch/sucrose non fermentable (SWI/SNF) chromatin remodeling complex. Accordingly, histamine or MS023 treatment could decrease ROS and NETs ex vivo, and ameliorated cardiac function and fibrosis, along with the reduced NETs in plasma in vivo. Together, our findings unveil the role of HDC in NETosis by histamine-H1R-ATP-SWI/SNF-PRMT1-ROS signaling and provide new biomarkers and targets for identifying and tuning the detrimental immune state in cardiovascular disease.
Keywords: Asymmetric demethylation arginine; HDC; Myocardial infarction; Neutrophil extracellular trap; PRMT1; Transcriptomics.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures




Similar articles
-
Histamine Deficiency Promotes Myofibroblasts Transformation from HDC-Expressing CD11b+ Myeloid Cells in Injured Hearts Post Myocardial Infarction.J Cardiovasc Transl Res. 2022 Jun;15(3):621-634. doi: 10.1007/s12265-021-10172-2. Epub 2021 Nov 3. J Cardiovasc Transl Res. 2022. PMID: 34734351
-
Histamine deficiency deteriorates LPS-induced periodontal diseases in a murine model via NLRP3/Caspase-1 pathway.Int Immunopharmacol. 2023 Feb;115:109630. doi: 10.1016/j.intimp.2022.109630. Epub 2022 Dec 24. Int Immunopharmacol. 2023. PMID: 36571917
-
Histamine deficiency facilitates coronary microthrombosis after myocardial infarction by increasing neutrophil-platelet interactions.J Cell Mol Med. 2020 Mar;24(6):3504-3520. doi: 10.1111/jcmm.15037. Epub 2020 Feb 16. J Cell Mol Med. 2020. PMID: 32064748 Free PMC article.
-
[Progress in mechanism of formation of neutrophil extracellular traps: Review].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020 Jun;36(6):561-564. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020. PMID: 32696748 Review. Chinese.
-
An exploratory look at NETosis in atherosclerosis.Intern Emerg Med. 2017 Feb;12(1):13-22. doi: 10.1007/s11739-016-1543-2. Epub 2016 Sep 21. Intern Emerg Med. 2017. PMID: 27655025 Review.
Cited by
-
Neutrophil extracellular traps in acute coronary syndrome.J Inflamm (Lond). 2023 May 10;20(1):17. doi: 10.1186/s12950-023-00344-z. J Inflamm (Lond). 2023. PMID: 37165396 Free PMC article. Review.
-
Endothelial PRMT7 prevents dysfunction, promotes revascularization and enhances cardiac recovery post-myocardial infarction.Exp Mol Med. 2025 Aug 5. doi: 10.1038/s12276-025-01517-x. Online ahead of print. Exp Mol Med. 2025. PMID: 40764455
-
MMP9High Neutrophils are Critical Mediators of Neutrophil Extracellular Traps Formation and Myocardial Ischemia/Reperfusion Injury.Adv Sci (Weinh). 2025 Jun;12(21):e2415205. doi: 10.1002/advs.202415205. Epub 2025 Mar 28. Adv Sci (Weinh). 2025. PMID: 40151877 Free PMC article.
-
Effect of inflammatory factors on myocardial infarction.BMC Cardiovasc Disord. 2024 Oct 7;24(1):538. doi: 10.1186/s12872-024-04122-4. BMC Cardiovasc Disord. 2024. PMID: 39375629 Free PMC article.
-
The cGAS-STING pathway in cardiovascular diseases: from basic research to clinical perspectives.Cell Biosci. 2024 May 8;14(1):58. doi: 10.1186/s13578-024-01242-4. Cell Biosci. 2024. PMID: 38720328 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

