Disruption of adipocyte HIF-1 α improves atherosclerosis through the inhibition of ceramide generation
- PMID: 35847503
- PMCID: PMC9279628
- DOI: 10.1016/j.apsb.2021.10.001
Disruption of adipocyte HIF-1 α improves atherosclerosis through the inhibition of ceramide generation
Abstract
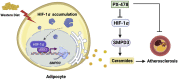
Atherosclerosis is a chronic multifactorial cardiovascular disease. Western diets have been reported to affect atherosclerosis through regulating adipose function. In high cholesterol diet-fed ApoE -/- mice, adipocyte HIF-1α deficiency or direct inhibition of HIF-1α by the selective pharmacological HIF-1α inhibitor PX-478 alleviates high cholesterol diet-induced atherosclerosis by reducing adipose ceramide generation, which lowers cholesterol levels and reduces inflammatory responses, resulting in improved dyslipidemia and atherogenesis. Smpd3, the gene encoding neutral sphingomyelinase, is identified as a new target gene directly regulated by HIF-1α that is involved in ceramide generation. Injection of lentivirus-SMPD3 in epididymal adipose tissue reverses the decrease in ceramides in adipocytes and eliminates the improvements on atherosclerosis in the adipocyte HIF-1α-deficient mice. Therefore, HIF-1α inhibition may constitute a novel approach to slow atherosclerotic progression.
Keywords: ACER2/3, alkaline ceramidase 2/3; APOE, apolipoprotein E; ARNT, aryl hydrocarbon nuclear translocator; Adipocyte; Atherosclerosis; CCL5, chemokine (C–C motif) ligand 5; CERS2/4/5/6, ceramide synthase 2/4/5/6; CXCL1, chemokine (C–X–C motif) ligand 1 protein; Ceramide; ChIP, chromatin immunoprecipitation; Cholesterol; CoCl2, cobalt(Ⅱ) chloride; DEGS1, delta(4)-desaturase, sphingolipid 1; EIF5, eukaryotic translation initiation factor 5; GFP, green fluorescent protein; HDL, high-density lipoprotein; HIF-1α; HIF-1α/2α/3α, hypoxia-inducible factor 1 alpha/2 alpha/3 alpha; HREs, HIF-response elements; IL-6/1β, interleukin-6/1β; Inflammatory responses; LDL, low-density lipoprotein; MAC-2, lectin, galactose binding, soluble 3; MCP-1, monocyte chemoattractant protein-1; PC, phosphatidylcholine; PLS-DA, partial least squares discriminant analysis; PX-478; SGMS1, sphingomyelin synthase 1; SM, sphingomyelin; SMPD1/2/3/4, sphingomyelin phosphodiesterase 1/2/3/4; SMPD3; SMase, sphingomyelinase; SPTLC1/2/3, serine palmitoyltransferase long chain base subunit 1/2/3; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; VIP, variable importance for the projection; VLDL, very low-density lipoprotein; eWAT, epididymal white adipose tissue.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures

Similar articles
-
Adipocyte Hypoxia-Inducible Factor 2α Suppresses Atherosclerosis by Promoting Adipose Ceramide Catabolism.Cell Metab. 2019 Nov 5;30(5):937-951.e5. doi: 10.1016/j.cmet.2019.09.016. Epub 2019 Oct 24. Cell Metab. 2019. PMID: 31668872
-
Endothelial Hypoxia-Inducible Factor-1α Promotes Atherosclerosis and Monocyte Recruitment by Upregulating MicroRNA-19a.Hypertension. 2015 Dec;66(6):1220-6. doi: 10.1161/HYPERTENSIONAHA.115.05886. Epub 2015 Oct 19. Hypertension. 2015. PMID: 26483345
-
Prolonged fasting activates hypoxia inducible factors-1α, -2α and -3α in a tissue-specific manner in northern elephant seal pups.Gene. 2013 Sep 10;526(2):155-63. doi: 10.1016/j.gene.2013.05.004. Epub 2013 May 22. Gene. 2013. PMID: 23707926 Free PMC article.
-
Adipocyte-Macrophage Cross-Talk in Obesity.Adv Exp Med Biol. 2017;960:327-343. doi: 10.1007/978-3-319-48382-5_14. Adv Exp Med Biol. 2017. PMID: 28585206 Review.
-
Hypoxia inducible factor pathways as targets for functional foods.J Agric Food Chem. 2005 May 18;53(10):3751-68. doi: 10.1021/jf0479719. J Agric Food Chem. 2005. PMID: 15884793 Review.
Cited by
-
Role of ceramides in diabetic foot ulcers (Review).Int J Mol Med. 2023 Mar;51(3):26. doi: 10.3892/ijmm.2023.5229. Epub 2023 Feb 17. Int J Mol Med. 2023. PMID: 36799149 Free PMC article. Review.
-
Puerarin Targets HIF-1α to Modulate Hypoxia-Related Sphingolipid Metabolism in Diabetic Hepatopathy via the SPTLC2/Ceramide Pathway.Pharmaceuticals (Basel). 2025 Mar 12;18(3):398. doi: 10.3390/ph18030398. Pharmaceuticals (Basel). 2025. PMID: 40143173 Free PMC article.
-
Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets.Signal Transduct Target Ther. 2023 Nov 20;8(1):431. doi: 10.1038/s41392-023-01652-9. Signal Transduct Target Ther. 2023. PMID: 37981648 Free PMC article. Review.
-
Unraveling the Roles of HIF-1, HO-1, GLUT-1 and GLUT-4 in Myocardial Protection.Int J Drug Discov Pharm. 2024 Sep;3(3):100016. doi: 10.53941/ijddp.2024.100016. Epub 2024 Aug 26. Int J Drug Discov Pharm. 2024. PMID: 40376262 Free PMC article.
-
Unraveling anti-atherosclerosis mechanism of anthocyanins from Xinjiang wild cherry plum (Prunus divaricata Ledeb) via network pharmacology and molecular docking.Bioresour Bioprocess. 2025 Jun 6;12(1):53. doi: 10.1186/s40643-025-00900-w. Bioresour Bioprocess. 2025. PMID: 40478326 Free PMC article.
References
-
- Bittencourt M.S. Beyond the stenosis paradigm: atherosclerosis burden on a risk prediction score for suspected chronic CAD. JACC Cardiovasc Imaging. 2021;14:451–453. - PubMed
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., et al. Heart disease and stroke statistics—2019 update: a report from the american heart association. Circulation. 2019;139:e56–e528. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

