In vitro and in vivo characterization of erythrosin B and derivatives against Zika virus
- PMID: 35847519
- PMCID: PMC9279632
- DOI: 10.1016/j.apsb.2021.10.017
In vitro and in vivo characterization of erythrosin B and derivatives against Zika virus
Abstract
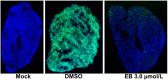
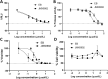
Zika virus (ZIKV) causes significant human diseases without specific therapy. Previously we found erythrosin B, an FDA-approved food additive, inhibited viral NS2B-NS3 interactions, leading to inhibition of ZIKV infection in cell culture. In this study, we performed pharmacokinetic and in vivo studies to demonstrate the efficacy of erythrosin B against ZIKV in 3D mini-brain organoid and mouse models. Our results showed that erythrosin B is very effective in abolishing ZIKV replication in the 3D organoid model. Although pharmacokinetics studies indicated that erythrosin B had a low absorption profile, mice challenged by a lethal dose of ZIKV showed a significantly improved survival rate upon oral administration of erythrosin B, compared to vehicle control. Limited structure-activity relationship studies indicated that most analogs of erythrosin B with modifications on the xanthene ring led to loss or reduction of inhibitory activities towards viral NS2B-NS3 interactions, protease activity and antiviral efficacy. In contrast, introducing chlorine substitutions on the isobenzofuran ring led to slightly increased activities, suggesting that the isobenzofuran ring is well tolerated for modifications. Cytotoxicity studies indicated that all derivatives are nontoxic to human cells. Overall, our studies demonstrated erythrosin B is an effective antiviral against ZIKV both in vitro and in vivo.
Keywords: AUC, area under he curve; Antiviral; DENV, dengue virus; DMSO, dimethyl sulfoxide; Dengue virus; EB, erythrosin B; Erythrosin B; FDA, US Food and Drug Administration; FRET, fluorescence resonance energy transfer; Flavivirus; NS, non-structural protein; ORF, open reading frame; PFU, plaque-forming unit; PK, pharmacokinetic; PP, polyprotein precursor; Protease inhibitor; SAR, structure−activity relationship; SLC, split luciferase complementation; UTR, untranslated region; WHO, World Health Organization; ZIKV, Zika virus; Zika virus; aa, amino acid; dpi, day post infection; ip, intraperitoneal.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures


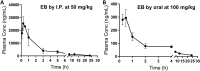

References
-
- Calvet G., Aguiar R.S., Melo A.S., Sampaio S.A., de Filippis I., Fabri A., et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653–660. - PubMed
-
- Thomas D.L., Sharp T.M., Torres J., Armstrong P.A., Munoz-Jordan J., Ryff K.R., et al. Local transmission of Zika virus—Puerto Rico, November 23, 2015−January 28, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:154–158. - PubMed
-
- Martines R.B., Bhatnagar J., Keating M.K., Silva-Flannery L., Muehlenbachs A., Gary J., et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses —Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–160. - PubMed
-
- Rodriguez-Morales A.J. Zika and microcephaly in Latin America: an emerging threat for pregnant travelers?. Trav Med Infect Dis. 2016;14:5–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

