Ameliorative Effects of Arctigenin on Pulmonary Fibrosis Induced by Bleomycin via the Antioxidant Activity
- PMID: 35847593
- PMCID: PMC9277162
- DOI: 10.1155/2022/3541731
Ameliorative Effects of Arctigenin on Pulmonary Fibrosis Induced by Bleomycin via the Antioxidant Activity
Abstract
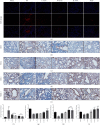
In this study, we evaluated the in vivo effect of arctigenin (ATG) on bleomycin-induced pulmonary fibrosis in mice and assessed the role of antioxidant activity. Hematoxylin and eosin (H&E) staining, the results of Masson's trichrome, and Sirius red staining showed that bleomycin induced obvious pathological changes and collagen deposition in the lung tissue of mice, which were effectively inhibited by ATG. Specifically, based on immunohistochemistry and western blot results, ATG inhibited the expression of fibrosis markers, such as collagen, fibronectin, and α-SMA. Moreover, ATG regulated reactive oxygen species (ROS), superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) in the lung tissue of pulmonary fibrosis mice and reduced the pressure of oxidative stress. ATG also regulated the TGF-β-induced expression of p-Akt, confirming that ATG can inhibit fibrosis through antioxidant activity modulation.
Copyright © 2022 Yueshang Wang et al.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




References
-
- Rogliani P., Calzetta L., Cavalli F., Matera M. G., Cazzola M. Pirfenidone, nintedanib and _N_ -acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulmonary Pharmacology & Therapeutics . 2016;40:95–103. doi: 10.1016/j.pupt.2016.07.009. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

