Activation of TLR-pathway to induce host Th1 immune response against visceral leishmaniasis: Involvement of galactosylated-flavonoids
- PMID: 35847617
- PMCID: PMC9284459
- DOI: 10.1016/j.heliyon.2022.e09868
Activation of TLR-pathway to induce host Th1 immune response against visceral leishmaniasis: Involvement of galactosylated-flavonoids
Abstract
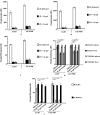
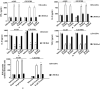
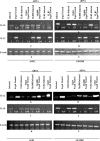
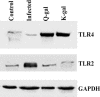
Immunotherapeutic strategies against visceral leishmaniasis (VL) are pertinent because of the emergence of resistance against existing chemotherapy, coupled with their toxicity and high costs. Various bioactive components with potential immunomodulatory activity, such as alkaloids, terpenes, saponins, flavonoids obtained primarily from medicinal plants, have been screened against different disease models. Reports suggested that glycans containing terminal β-galactose can skew host immune response towards Th1 by engaging TLRs. In this study, two synthesized terminal galactose-containing flavones, Quercetin 3-d-galactoside (Q-gal) and Kaempferol 3-O-d-galactoside (K-gal), are profiled in terms of inducing host protective Th1 response in both in vitro & in vivo animal models of experimental VL individually against antimony-resistant & antimony-susceptible Leishmania donovani. Further, we explored that both Q-gal and K-gal induce TLR4 mediated Th1 response to encounter VL. Molecular docking analysis also suggested strong interaction with TLR4 for both the galactosides, with a slightly better binding potential towards Q-gal. Treatment with both Q-gal and K-gal showed significant antileishmanial efficacy. Each considerably diminished the liver and splenic parasite burden 60 days after post-infection (>90% in AG83 infected mice and >87% in GE1F8R infected mice) when administered at a 5 mg/kg/day body-weight dose for ten consecutive days. However, the treatments failed to clear the parasites in the TLR4 deficient C3H/HeJ mice. Treatment with these compounds favors the elevation of TLR4 dependent host protective Th1 cytokines and suppression of disease-promoting IL-10. Q-gal and K-gal also triggered sufficient ROS generation in macrophages to kill intracellular parasites directly.
Keywords: Antileishmanial; Galactosylated-Flavonoids; Reactive oxygen species; TLR-4; Th1 response; Visceral leishmaniasis.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures










Similar articles
-
Asiaticoside induces tumour-necrosis-factor-α-mediated nitric oxide production to cure experimental visceral leishmaniasis caused by antimony-susceptible and -resistant Leishmania donovani strains.J Antimicrob Chemother. 2012 Apr;67(4):910-20. doi: 10.1093/jac/dkr575. Epub 2012 Jan 18. J Antimicrob Chemother. 2012. PMID: 22258930
-
Co-administration of glycyrrhizic acid with the antileishmanial drug sodium antimony gluconate (SAG) cures SAG-resistant visceral leishmaniasis.Int J Antimicrob Agents. 2015 Mar;45(3):268-77. doi: 10.1016/j.ijantimicag.2014.10.023. Epub 2014 Dec 8. Int J Antimicrob Agents. 2015. PMID: 25600891
-
Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis.J Immunol. 2005 Jun 1;174(11):7160-71. doi: 10.4049/jimmunol.174.11.7160. J Immunol. 2005. PMID: 15905560
-
Live Attenuated Leishmania donovani Centrin Knock Out Parasites Generate Non-inferior Protective Immune Response in Aged Mice against Visceral Leishmaniasis.PLoS Negl Trop Dis. 2016 Aug 31;10(8):e0004963. doi: 10.1371/journal.pntd.0004963. eCollection 2016 Aug. PLoS Negl Trop Dis. 2016. PMID: 27580076 Free PMC article.
-
Eugenol derived immunomodulatory molecules against visceral leishmaniasis.Eur J Med Chem. 2017 Oct 20;139:503-518. doi: 10.1016/j.ejmech.2017.08.030. Epub 2017 Aug 12. Eur J Med Chem. 2017. PMID: 28826085
References
-
- Aderem A., Ulevitch R.J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–787. - PubMed
-
- Ashour D.S. Toll-like receptor signaling in parasitic infections. Expet Rev. Clin. Immunol. 2015;11(6):771–780. - PubMed
-
- Basu R., Bhaumik S., Basu J.M., Naskar K., De T., Roy S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and-resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1-and Th2-like responses in visceral leishmaniasis. J. Immunol. 2005;174(11):7160–7171. - PubMed
-
- Bhaumik S.K., Naskar K., De T. Complete protection against experimental visceral leishmaniasis with complete soluble antigen from attenuated Leishmania donovani promastigotes involves Th1-immunity and down-regulation of IL-10. Eur. J. Immunol. 2009;39(8):2146–2160. - PubMed
-
- Caielli S., Conforti-Andreoni C., Di Pietro C., Usuelli V., Badami E., Malosio M.L., et al. On/off TLR signaling decides proinflammatory or tolerogenic dendritic cell maturation upon CD1d-mediated interaction with invariant NKT cells. J. Immunol. 2010;185(12):7317–7329. - PubMed
LinkOut - more resources
Full Text Sources

