A pilot clinical phase II trial MemSID: Acute and durable changes of red blood cells of sickle cell disease patients on memantine treatment
- PMID: 35847705
- PMCID: PMC9175962
- DOI: 10.1002/jha2.11
A pilot clinical phase II trial MemSID: Acute and durable changes of red blood cells of sickle cell disease patients on memantine treatment
Abstract
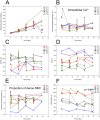
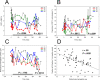
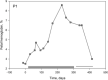
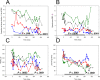
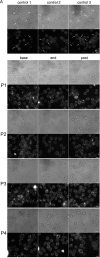
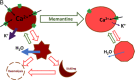
An increase in abundance and activity of N-methyl D-aspartate receptors (NMDAR) was previously reported for red blood cells (RBCs) of sickle cell disease (SCD) patients. Increased Ca2+ uptake through the receptor supported dehydration and RBC damage. In a pilot phase IIa-b clinical trial MemSID, memantine, a blocker of NMDAR, was used for treatment of four patients for 12 months. Two more patients that have enrolled into the study did not finish it. One of them had psychotic event following the involuntary overdose of the drug, whereas the other had vertigo and could not comply to the trial visits schedule. Acute and durable responses of RBCs of SCD patients to daily oral administration of memantine were monitored. Markers of RBC turnover, changes in cell density, and alterations in ion handling and RBC morphology were assessed. Acute transient shifts in intracellular Ca2+, volume and density, and reduction in plasma lactate dehydrogenate activity were observed already within the first month of treatment. Durable effects of memantine included (a) decrease in reticulocyte counts, (b) reduction in reticulocyte hemoglobinization, (c) advanced membrane maturation and its stabilization as follows from reduction in the number of NMDAR per cell and reduction in hemolysis, and (iv) rehydration and decrease in K+ leakage from patients' RBC. Memantine therapy resulted in reduction in number of cells with sickle morphology that was sustained at least over 2 months after therapy was stopped indicating an improvement in RBC longevity.
© 2020 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest. The University of Zurich holds the patent to use memantine against sickle cell disease.
Figures



References
-
- Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;377:305. - PubMed
-
- Rees DC, Williams TN, Gladwin MT. Sickle‐cell disease. Lancet. 2010;376:2018–31. - PubMed
-
- Bender MA. Sickle cell disease. In: GeneReviews(R) (ed. by Adam MP, Ardinger HH,Pagon RA, Wallace SE, Bean LJH, Stephens K & Amemiya A), Seattle, WA: University of Washington; 1993. - PubMed
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311–23. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
