Diagnostic and molecular testing patterns in patients with newly diagnosed acute myeloid leukemia in the Connect® MDS/AML Disease Registry
- PMID: 35847712
- PMCID: PMC9176048
- DOI: 10.1002/jha2.16
Diagnostic and molecular testing patterns in patients with newly diagnosed acute myeloid leukemia in the Connect® MDS/AML Disease Registry
Abstract
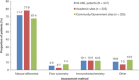
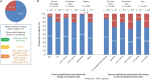
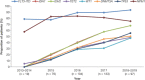
Diagnostic and molecular genetic testing are key in advancing the treatment of acute myeloid leukemia (AML), yet little is known about testing patterns outside of clinical trials, especially in older patients. We analyzed diagnostic and molecular testing patterns over time in 565 patients aged ≥ 55 years with newly diagnosed AML enrolled in the Connect® MDS/AML Disease Registry (NCT01688011) in the United States. Diagnostic data were recorded at enrolment and compared with published guidelines. The percentage of bone marrow blasts was reported for 82.1% of patients, and cellularity was the most commonly reported bone marrow morphological feature. Flow cytometry, karyotyping, molecular testing, and fluorescence in situ hybridization were performed in 98.8%, 95.4%, 75.9%, and 75.7% of patients, respectively. Molecular testing was done more frequently at academic than community/government sites (84.3% vs 70.2%; P < .001). Enrolment to the Registry after 2016 was significantly associated with molecular testing at academic sites (odds ratio [OR] 2.59; P = .023) and at community/government sites (OR 4.85; P < .001) in logistic regression analyses. Better understanding of practice patterns may identify unmet needs and inform institutional protocols regarding the diagnosis of patients with AML.
Keywords: acute myeloid leukemia; diagnostic testing; leukemia diagnosis; leukemia therapy; molecular testing; registry.
© 2020 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
DAP: AbbVie, Bristol‐Myers Squibb, Daiichi Sankyo – advisory board member and consultancy; Agios, Forty Seven, Pfizer – advisory board member; Takeda – consultancy; Glycomimetics – data safety and monitoring committee. TIG: Bristol‐Myers Squibb – consultancy; MA: Bristol‐Myers Squibb – advisory board, AbbVie, Bristol‐Myers Squibb, Gilead, Seattle Genetrix, Takeda – speaker panel; CRC, JPM, GGM: no conflicts to disclose; RB: AbbVie, Astex Daiichi Sankyo, Forty Seven, NeoGenomics – consultancy; Bristol‐Myers Squibb – consultancy, honoraria, research funding; Xian‐Janssen – honoraria; KF: Bristol‐Myers Squibb – advisory board member. DLG: AbbVie – consultancy; Alexion – speakers bureau; Astellas, Bristol‐Myers Squibb – advisory board member. RSK: Alexion, Jazz Pharmaceuticals, Novartis – speakers bureau; Agios, Bristol‐Myers Squibb, Daiichi Sankyo, Inc., Incyte, Janssen, Pfizer – consultancy. DAR: Allergan, Amgen, Bristol‐Myers Squibb, Takeda – research funding and consultancy. GJR: AbbVie, Actinum, Agios, Amphivena, Argenx, Astex, Astellas, Bayer, Bristol‐Myers Squibb, Celltrion, Daiichi Sankyo, Eisai, Janssen, Jazz Pharmaceuticals, Novartis, MEI Pharma, Orsenix, Otsuka, Pfizer, Roche/Genentech, Sandoz, Takeda, Trovgene – consultancy, advisory board or data and safety monitoring committee; Cellectis – research funding. MRS: AbbVie – advisory board member, consulting; Boehringer Ingelheim – patents and royalties; Bristol‐Myers Squibb, Selvita – advisory board member; Incyte – advisory board member, research funding; Karyopharm – advisory board member, consultancy, equity ownership; Sunesis – research funding; Takeda, TG Therapeutics – advisory board member, research funding. BLS: Agios – speakers bureau; Alexion, Bristol‐Myers Squibb – advisory board member, consultancy, speakers bureau; Incyte – advisory board member, speakers bureau; Novartis – research funding. MAS: Bristol‐Myers Squibb, Pfizer, Takeda/Millenium – consulting. MAT: Adaptive, Bristol‐Myers Squibb, Doximity, GlaxoSmithKline, Strata Oncology, Syapse Precision Medicine Council, VIA Oncology, UpToDate – consultancy; Doximity – equity; AbbVie, Bristol Myers‐Squibb, CRAB CTC, Denovo, Hoosier Research Network, Lilly, LynxBio, Stata Oncology, Takeda, TG Therapeutics – institutional research funding; SEK: Agios and Bristol‐Myers Squibb – consultancy. CUL, MN, ASS, PK: Bristol‐Myers Squibb ‐ equity and employment. EDF: Bristol‐Myers Squibb – employment. DPS: Astex, Bristol‐Myers Squibb, Onconova, Pfizer, StemLine, Summer Road, Takeda – consultancy. HPE: Agios, Bristol‐Myers Squibb, Jazz Pharmaceuticals, Incyte, Novartis – speakers bureau; AbbVie, Agios, Amgen, Astellas, Bristol‐Myers Squibb, Daiichi Sankyo, Glycomimetics, ImmunoGen, Incyte, Jazz, MacroGenics, Novartis, Pfizer, Seattle Genetics – consultancy; AbbVie, Daiichi Sankyo, ImmunoGen, Macrogenics – research funding; Glycomimetics – data safety and monitoring committee; Covance – independent review committee.
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin. 2019;69:7–34. - PubMed
-
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. (eds). SEER Cancer Statistics Review, 1975‐2015, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. Accessed 17 September 2019.
-
- Arber DA, Borowitz MJ, Cessna M., Etzell J, Foucar K, Hasserjian RP, et al. Initial diagnostic workup of acute leukemia: guideline from the College of American Pathologists and the American Society of Hematology. Arch Pathol Lab Med. 2017;141:1342–93. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
