Immune reconstitution in children following chemotherapy for acute leukemia
- PMID: 35847713
- PMCID: PMC9176016
- DOI: 10.1002/jha2.27
Immune reconstitution in children following chemotherapy for acute leukemia
Abstract
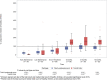
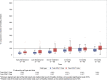
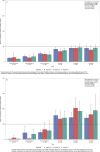
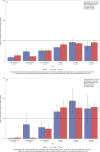
Although survival rates for pediatric acute lymphoblastic leukemia are now excellent, this is at the expense of prolonged chemotherapy regimens. We report the long-term immune effects in children treated according to the UK Medical Research Council UKALL 2003 protocol. Peripheral blood lymphocyte subsets and immunoglobulin levels were studied in 116 participants, at six time points, during and for 18-month following treatment, with 30-39 patients analyzed at each time point. Total lymphocytes were reduced during maintenance chemotherapy and remained low 18 months following treatment completion. CD4 T cells remained significantly reduced 18 months after treatment, but CD8 cells and natural killer cells recovered to normal values. The fall in naïve B-cell numbers during maintenance was most marked, but numbers recovered rapidly after cessation of treatment. Memory B cells, particularly nonclass-switched memory B cells, remained below normal levels 18 months following treatment. All immunoglobulin subclasses were reduced during treatment compared to normal values, with IgM levels most affected. This study demonstrates that immune reconstitution differs between lymphocyte compartments. Although total B-cell numbers recover rapidly, disruption of memory/naïve balance persists and T-cell compartment persist at 18 months. This highlights the impact of modern chemotherapy regimens on immunity, and thus, infectious susceptibility and response to immunization.
© 2020 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Figures

References
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541‐52. - PubMed
-
- Bonaventure A, Harewood R, Stiller CA, et al. Worldwide comparison of survival from childhood leukaemia for 1995–2009, by subtype, age, and sex (CONCORD‐2): a population‐based study of individual data for 89 828 children from 198 registries in 53 countries. Lancet Haematol. 2017;4(5):e202‐17. - PMC - PubMed
-
- Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low‐risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199‐209. - PubMed
-
- Kosmidis S, Baka M, Bouhoutsou D, et al. Longitudinal assessment of immunological status and rate of immune recovery following treatment in children with ALL. Pediatr Blood Cancer. 2008;50(3):528‐32. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
