Yifei Sanjie Formula Treats Chronic Obstructive Pulmonary Disease by Remodeling Pulmonary Microbiota
- PMID: 35847812
- PMCID: PMC9277004
- DOI: 10.3389/fmed.2022.927607
Yifei Sanjie Formula Treats Chronic Obstructive Pulmonary Disease by Remodeling Pulmonary Microbiota
Abstract
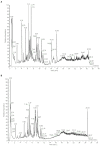
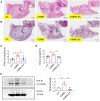
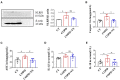
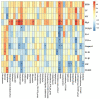
Chronic obstructive pulmonary disease (COPD) is one of the most common pulmonary diseases. Evidence suggests that dysbiosis of pulmonary microbiota leads to the COPD pathological process. Yifei Sanjie Formula (YS) is widely used to treat diseases in respiratory systems, yet little is known about its mechanisms. In the present study, we first established the fingerprint of YS as the background for UHPLC-QTOF-MS. Components were detected, including alkaloids, amino acid derivatives, phenylpropanoids, flavonoids, terpenoids, organic acids, phenols, and the like. The therapeutic effect of YS on COPD was evaluated, and the pulmonary function and ventilatory dysfunction (EF50, TV, and MV) were improved after the administration of YS. Further, the influx of lymphocytes was inhibited in pulmonary parenchyma, accompanied by down-regulation of inflammation cytokines via the NLRP3/caspase-1/IL-1β signaling pathway. The severity of pulmonary pathological damage was reversed. Disturbed pulmonary microbiota was discovered to involve an increased relative abundance of Ralstonia and Mycoplasma and a decreased relative abundance of Lactobacillus and Bacteroides in COPD animals. However, the subversive effect was shown. The abundance and diversity of pulmonary microflora were remodeled, especially increasing beneficial genua Lactobacillus and Bacteroides, as well as downregulating pathogenic genua Ralstonia and Mycoplasma in the YS group. Environmental factor correlation analysis showed that growing pulmonary microbiota was positively correlated with the inflammatory factor, referring to Ralstonia and Mycoplasma, as well as negatively correlated with the inflammatory factor, referring to Lactobacillus and Bacteroides. These results suggest that the effects of YS involved remodeling lung microbes and anti-inflammatory signal pathways, revealing that intervention microbiota and an anti-inflammatory may be a potential therapeutic strategy for COPD.
Keywords: NLRP3/caspase-1/IL-1β signaling pathway; Yifei Sanjie Formula; chronic obstructive pulmonary disease; inflammation; pulmonary microbiota.
Copyright © 2022 Wu, Meng, Qiao, Li, Zhang, Jia, Xing, Li, Yuan and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The treatment of Qibai Pingfei Capsule on chronic obstructive pulmonary disease may be mediated by Th17/Treg balance and gut-lung axis microbiota.J Transl Med. 2022 Jun 21;20(1):281. doi: 10.1186/s12967-022-03481-w. J Transl Med. 2022. PMID: 35729584 Free PMC article.
-
Links Between Inflammatory Bowel Disease and Chronic Obstructive Pulmonary Disease.Front Immunol. 2020 Sep 11;11:2144. doi: 10.3389/fimmu.2020.02144. eCollection 2020. Front Immunol. 2020. PMID: 33042125 Free PMC article. Review.
-
The inhibitory effect of Yupingfengsan and Siwutang compound formula on inflammation and oxidative stress in COPD rats.Pak J Pharm Sci. 2020 Jul;33(4):1493-1501. Pak J Pharm Sci. 2020. PMID: 33583779
-
Effective-component compatibility of Bufei Yishen formula II inhibits mucus hypersecretion of chronic obstructive pulmonary disease rats by regulating EGFR/PI3K/mTOR signaling.J Ethnopharmacol. 2020 Jul 15;257:112796. doi: 10.1016/j.jep.2020.112796. Epub 2020 Apr 25. J Ethnopharmacol. 2020. PMID: 32344236
-
Role of pulmonary microorganisms in the development of chronic obstructive pulmonary disease.Crit Rev Microbiol. 2021 Feb;47(1):1-12. doi: 10.1080/1040841X.2020.1830748. Epub 2020 Oct 10. Crit Rev Microbiol. 2021. PMID: 33040638 Review.
References
LinkOut - more resources
Full Text Sources

