Mouse Models of Hepatocellular Carcinoma: Classification, Advancement, and Application
- PMID: 35847898
- PMCID: PMC9279915
- DOI: 10.3389/fonc.2022.902820
Mouse Models of Hepatocellular Carcinoma: Classification, Advancement, and Application
Abstract
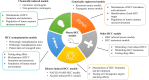
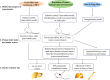
Hepatocellular carcinoma (HCC) is the subtype of liver cancer with the highest incidence, which is a heterogeneous malignancy with increasing incidence rate and high mortality. For ethical reasons, it is essential to validate medical clinical trials for HCC in animal models before further consideration on humans. Therefore, appropriate models for the study of the pathogenesis of the disease and related treatment methods are necessary. For tumor research, mouse models are the most commonly used and effective in vivo model, which is closer to the real-life environment, and the repeated experiments performed on it are closer to the real situation. Several mouse models of HCC have been developed with different mouse strains, cell lines, tumor sites, and tumor formation methods. In this review, we mainly introduce some mouse HCC models, including induced model, gene-edited model, HCC transplantation model, and other mouse HCC models, and discuss how to choose the appropriate model according to the purpose of the experiments.
Keywords: gene-edited mice; hepatocellular carcinoma; metastasis; mouse model; transplantation model.
Copyright © 2022 Liu, Huang, Ru, Wang, Zhang, Chen and Chu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Intrahepatic Tissue Implantation Represents a Favorable Approach for Establishing Orthotopic Transplantation Hepatocellular Carcinoma Mouse Models.PLoS One. 2016 Jan 29;11(1):e0148263. doi: 10.1371/journal.pone.0148263. eCollection 2016. PLoS One. 2016. PMID: 26824903 Free PMC article.
-
Multiparametric Analyses of Hepatocellular Carcinoma Somatic Mouse Models and Their Associated Tumor Microenvironment.Curr Protoc. 2021 Jun;1(6):e147. doi: 10.1002/cpz1.147. Curr Protoc. 2021. PMID: 34101385
-
Notch activity characterizes a common hepatocellular carcinoma subtype with unique molecular and clinicopathologic features.J Hepatol. 2021 Mar;74(3):613-626. doi: 10.1016/j.jhep.2020.09.032. Epub 2020 Oct 8. J Hepatol. 2021. PMID: 33038431 Free PMC article.
-
Preclinical mouse models of hepatocellular carcinoma: An overview and update.Exp Cell Res. 2022 Mar 15;412(2):113042. doi: 10.1016/j.yexcr.2022.113042. Epub 2022 Jan 29. Exp Cell Res. 2022. PMID: 35101391 Review.
-
Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research.Nat Rev Gastroenterol Hepatol. 2018 Sep;15(9):536-554. doi: 10.1038/s41575-018-0033-6. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29904153 Review.
Cited by
-
Mechanistically based blood proteomic markers in the TGF-β pathway stratify risk of hepatocellular cancer in patients with cirrhosis.Genes Cancer. 2024 Feb 1;15:1-14. doi: 10.18632/genesandcancer.234. eCollection 2024. Genes Cancer. 2024. PMID: 38323119 Free PMC article.
-
A novel phosphodiesterase target as a therapeutic approach: inhibiting DEN-induced hepatocellular carcinoma progression.EXCLI J. 2025 Mar 7;24:407-429. doi: 10.17179/excli2024-7941. eCollection 2025. EXCLI J. 2025. PMID: 40166422 Free PMC article. Review.
-
Plasmodium infection downregulates hypoxia‑inducible factor 1α expression to suppress the vascularization and tumorigenesis of liver cancer.Oncol Lett. 2024 Oct 14;28(6):604. doi: 10.3892/ol.2024.14737. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39483968 Free PMC article.
-
Resistance against the development of diethylnitrosamine-induced hepatocellular carcinoma in female C3H mice: an experimental model.Exp Anim. 2024 Oct 23;73(4):399-411. doi: 10.1538/expanim.23-0149. Epub 2024 Aug 3. Exp Anim. 2024. PMID: 39098024 Free PMC article.
-
Improving immunotherapy for the treatment of hepatocellular carcinoma: learning from patients and preclinical models.Gut Liver. 2025 Apr 3;2(1):s44355-025-00018-y. doi: 10.1038/s44355-025-00018-y. Gut Liver. 2025. PMID: 40626281 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

