Cu-Hemin Nanosheets and Indocyanine Green Co-Loaded Hydrogel for Photothermal Therapy and Amplified Photodynamic Therapy
- PMID: 35847901
- PMCID: PMC9280130
- DOI: 10.3389/fonc.2022.918416
Cu-Hemin Nanosheets and Indocyanine Green Co-Loaded Hydrogel for Photothermal Therapy and Amplified Photodynamic Therapy
Abstract
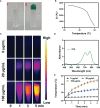
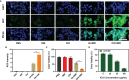
Near-infrared (NIR) organic small molecule indocyanine green (ICG) could respond well to 808 nm laser to promote local high temperature and ROS generation for realizing photothermal therapy (PTT)/photodynamic therapy (PDT). However, the high content of GSH in the tumor microenvironment (TME) limited the further therapeutic performance of ICG. Herein, injectable agarose in situ forming NIR-responsive hydrogels (CIH) incorporating Cu-Hemin and ICG were prepared for the first time. When CIH system was located to the tumor tissue through local injection, the ICG in the hydrogel could efficiently convert the light energy emitted by the 808 nm laser into thermal energy, resulting in the heating and softening of the hydrogel matrix, which releases the Cu-Hemin. Then, the over-expressed GSH in the TME could also down-regulated by Cu-Hemin, which amplified ICG-mediated PDT. In vivo experiments validated that ICG-based PDT/PTT and Cu-Hemin-mediated glutathione depletion could eliminate cancer tissues with admirable safety. This hydrogel-based GSH-depletion strategy is instructive to improve the objective response rate of PDT.
Keywords: glutathione; hydrogel; indocyanine green; photodynamic therapy; photothermal therapy.
Copyright © 2022 Zhu, Wang, Liu, Lyu and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Indocyanine Green-Loaded Silver Nanoparticle@Polyaniline Core/Shell Theranostic Nanocomposites for Photoacoustic/Near-Infrared Fluorescence Imaging-Guided and Single-Light-Triggered Photothermal and Photodynamic Therapy.ACS Appl Mater Interfaces. 2016 Dec 28;8(51):34991-35003. doi: 10.1021/acsami.6b11262. Epub 2016 Dec 13. ACS Appl Mater Interfaces. 2016. PMID: 27957854
-
Calcium-peroxide-mediated cascades of oxygen production and glutathione consumption induced efficient photodynamic and photothermal synergistic therapy.J Mater Chem B. 2023 Mar 30;11(13):2937-2945. doi: 10.1039/d2tb02776c. J Mater Chem B. 2023. PMID: 36912360
-
A near infrared-modulated thermosensitive hydrogel for stabilization of indocyanine green and combinatorial anticancer phototherapy.Biomater Sci. 2019 Mar 26;7(4):1705-1715. doi: 10.1039/c8bm01541d. Biomater Sci. 2019. PMID: 30758351
-
Recent Progress of Copper-Based Nanomaterials in Tumor-Targeted Photothermal Therapy/Photodynamic Therapy.Pharmaceutics. 2023 Sep 7;15(9):2293. doi: 10.3390/pharmaceutics15092293. Pharmaceutics. 2023. PMID: 37765262 Free PMC article. Review.
-
Metal-organic frameworks-loaded indocyanine green for enhanced phototherapy: a comprehensive review.Front Bioeng Biotechnol. 2025 May 27;13:1601476. doi: 10.3389/fbioe.2025.1601476. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40497249 Free PMC article. Review.
Cited by
-
Navigating heme pathways: the breach of heme oxygenase and hemin in breast cancer.Mol Cell Biochem. 2025 Mar;480(3):1495-1518. doi: 10.1007/s11010-024-05119-5. Epub 2024 Sep 17. Mol Cell Biochem. 2025. PMID: 39287890 Free PMC article. Review.
-
In situ thermal-responsive hydrogels for combined photothermal therapy and chemotherapy of pancreatic cancer.RSC Adv. 2025 Apr 24;15(17):13119-13126. doi: 10.1039/d5ra00371g. eCollection 2025 Apr 22. RSC Adv. 2025. PMID: 40275869 Free PMC article.
-
Photothermal therapy using graphene quantum dots.APL Bioeng. 2023 Aug 21;7(3):031502. doi: 10.1063/5.0160324. eCollection 2023 Sep. APL Bioeng. 2023. PMID: 37614868 Free PMC article. Review.
References
-
- Ding K, Zheng C, Sun L, Liu X, Yin Y, Wang L. NIR Light-Induced Tumor Phototherapy Using ICG Delivery System Based on Platelet-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles. Chin Chem Lett (2020) 31(5):1168–72. doi: 10.1016/j.cclet.2019.10.040 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

