Aquaporins in Cancer Biology
- PMID: 35847914
- PMCID: PMC9278817
- DOI: 10.3389/fonc.2022.782829
Aquaporins in Cancer Biology
Abstract
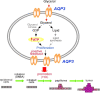
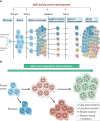
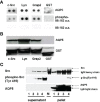
Aquaporins (AQPs) are a family of transmembrane water channel proteins, which were initially characterized as a novel protein family that plays a vital role in transcellular and transepithelial water movement. AQP1, AQP2, AQP4, AQP5, and AQP8 are primarily water selective, whereas AQP3, AQP7, AQP9, and AQP10 (called "aqua-glyceroporins") also transport glycerol and other small solutes. Recently, multiple reports have suggested that AQPs have important roles in cancer cell growth, migration, invasion, and angiogenesis, each of which is important in human carcinogenesis. Here, we review recent data concerning the involvement of AQPs in tumor growth, angiogenesis, and metastasis and explore the expression profiles from various resected cancer samples to further dissect the underlying molecular mechanisms. Moreover, we discuss the potential role of AQPs during the development of genomic instability and performed modeling to describe the integration of binding between AQPs with various SH3 domain binning adaptor molecules. Throughout review and discussion of numerous reports, we have tried to provide key evidence that AQPs play key roles in tumor biology, which may provide a unique opportunity in designing a novel class of anti-tumor agents.
Keywords: AQP expression; AQP in carcinogenesis; genomic instability; glycerol channel; hallmarks of cancer; targeted therapy; water channel.
Copyright © 2022 Moon, Moon and Kang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

