Acute Myeloid Leukaemia Drives Metabolic Changes in the Bone Marrow Niche
- PMID: 35847950
- PMCID: PMC9277016
- DOI: 10.3389/fonc.2022.924567
Acute Myeloid Leukaemia Drives Metabolic Changes in the Bone Marrow Niche
Abstract
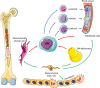
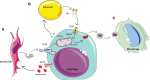
Acute myeloid leukaemia (AML) is a highly proliferative cancer characterised by infiltration of immature haematopoietic cells in the bone marrow (BM). AML predominantly affects older people and outcomes, particularly in this difficult to treat population remain poor, in part due to inadequate response to therapy, and treatment toxicity. Normal haematopoiesis is supported by numerous support cells within the BM microenvironment or niche, including adipocytes, stromal cells and endothelial cells. In steady state haematopoiesis, haematopoietic stem cells (HSCs) primarily acquire ATP through glycolysis. However, during stress-responses HSCs rapidly transition to oxidative phosphorylation, enabled by mitochondrial plasticity. Historically it was thought that cancer cells preferentially used glycolysis for ATP production, however recently it has become evident that many cancers, including AML primarily use the TCA cycle and oxidative phosphorylation for rapid proliferation. AML cells hijack the stress-response pathways of their non-malignant counterparts, utilising mitochondrial changes to drive expansion. In addition, amino acids are also utilised by leukaemic stem cells to aid their metabolic output. Together, these processes allow AML cells to maximise their ATP production, using multiple metabolites and fuelling rapid cell turnover which is a hallmark of the disease. This review of AML derived changes in the BM niche, which enable enhanced metabolism, will consider the important pathways and discuss future challenges with a view to understanding how AML cells are able to hijack metabolic pathways and how we may elucidate new targets for potential therapies.
Keywords: acute myeloid leukaemia; adipocytes; bone marrow niche; free fattty acids; metabolism.
Copyright © 2022 Maynard, Hellmich, Bowles and Rushworth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

