Librational Dynamics of Spin-Labeled Membranes at Cryogenic Temperatures From Echo-Detected ED-EPR Spectra
- PMID: 35847982
- PMCID: PMC9277068
- DOI: 10.3389/fmolb.2022.923794
Librational Dynamics of Spin-Labeled Membranes at Cryogenic Temperatures From Echo-Detected ED-EPR Spectra
Abstract
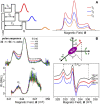
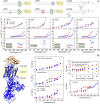
Methods of electron spin echo of pulse electron paramagnetic resonance (EPR) spectroscopy are increasingly employed to investigate biophysical properties of nitroxide-labeled biosystems at cryogenic temperatures. Two-pulse echo-detected ED-spectra have proven to be valuable tools to describe the librational dynamics in the low-temperature phases of both lipids and proteins in membranes. The motional parameter, , given by the product of the mean-square angular amplitude, , and the rotational correlation time, , of the motion, is readily determined from the nitroxide ED-spectra as well as from the W-relaxation rate curves. An independent evaluation of is obtained from the motionally averaged 14N-hyperfine splitting separation in the continuous wave cw-EPR spectra. Finally, the rotational correlation time can be estimated by combining ED- and cw-EPR data. In this mini-review, results on the librational dynamics in model and natural membranes are illustrated.
Keywords: Na, K-ATPase; echo-detected ED-spectra; electron paramagnetic resonance; electron spin echo; librations; model membranes; spin label.
Copyright © 2022 Bartucci and Aloi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

