Real-world use and outcomes of dolutegravir-containing antiretroviral therapy in HIV and tuberculosis co-infection: a site survey and cohort study in sub-Saharan Africa
- PMID: 35848120
- PMCID: PMC9289708
- DOI: 10.1002/jia2.25961
Real-world use and outcomes of dolutegravir-containing antiretroviral therapy in HIV and tuberculosis co-infection: a site survey and cohort study in sub-Saharan Africa
Abstract
Introduction: Dolutegravir is being scaled up globally as part of antiretroviral therapy (ART), but for people with HIV and tuberculosis co-infection, its use is complicated by a drug-drug interaction with rifampicin requiring an additional daily dose of dolutegravir. This represents a disadvantage over efavirenz, which does not have a major drug-drug interaction with rifampicin. We sought to describe HIV clinic practices for prescribing concomitant dolutegravir and rifampicin, and characterize virologic outcomes among patients with tuberculosis co-infection receiving dolutegravir or efavirenz.
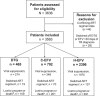
Methods: Within the four sub-Saharan Africa regions of the International epidemiology Databases to Evaluate AIDS consortium, we conducted a site survey (2021) and a cohort study (2015-2021). The cohort study used routine clinical data and included patients newly initiating or already receiving dolutegravir or efavirenz at the time of tuberculosis diagnosis. Patients were followed from tuberculosis diagnosis until viral suppression (<1000 copies/ml), a competing event (switching ART regimen; loss to program/death) or administrative censoring at 12 months.
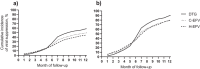
Results: In the survey, 86 of 90 (96%) HIV clinics in 18 countries reported prescribing dolutegravir to patients who were receiving rifampicin as part of tuberculosis treatment, with 77 (90%) reporting that they use twice-daily dosing of dolutegravir, of which 74 (96%) reported having 50 mg tablets available to accommodate twice-daily dosing. The cohort study included 3563 patients in 11 countries, with 67% newly or recently initiating ART. Among patients receiving dolutegravir (n = 465), the cumulative incidence of viral suppression was 58.9% (95% confidence interval [CI]: 54.3-63.3%), switching ART regimen was 4.1% (95% CI: 2.6-6.2%) and loss to program/death was 23.4% (95% CI: 19.7-27.4%). Patients receiving dolutegravir had improved viral suppression compared with patients receiving efavirenz who had a tuberculosis diagnosis before site dolutegravir availability (adjusted subdistribution hazard ratio [aSHR]: 1.47, 95% CI: 1.28-1.68) and after site dolutegravir availability (aSHR 1.28, 95% CI: 1.08-1.51).
Conclusions: At a programmatic level, dolutegravir was being widely prescribed in sub-Saharan Africa for people with HIV and tuberculosis co-infection with a dose adjustment for the drug-drug interaction with rifampicin. Despite this more complex regimen, our cohort study revealed that dolutegravir did not negatively impact viral suppression.
Keywords: HIV integrase inhibitors; antiretroviral agents; antitubercular agents; drug interactions; observational study; rifampin.
© 2022 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- World Health Organization . Update of recommendations on first‐ and second‐line antiretroviral regimens: policy brief. 2019. [cited 2022 March 11]. Available from: https://www.who.int/hiv/pub/arv/arv‐update‐2019‐policy/en/.
-
- World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery, and monitoring: recommendations for a public health approach. 2019. [cited 2022 March 11]. Available from: https://www.who.int/publications/i/item/9789240031593. - PubMed
-
- Clinton Health Access Initiative . HIV Market Report 2020; Issue 11. 2020. [cited 2022 March 11]. Available from: https://www.clintonhealthaccess.org/the‐state‐of‐the‐hiv‐market‐in‐low‐a....
-
- Phillips AN, Bansi‐Matharu L, Venter F, Havlir D, Pozniak A, Kuritzkes DR, et al. Updated assessment of risks and benefits of dolutegravir versus efavirenz in new antiretroviral treatment initiators in sub‐Saharan Africa: modelling to inform treatment guidelines. Lancet HIV. 2020;7(3):e193–200. - PMC - PubMed

