Tenofovir disoproxil fumarate and coronavirus disease 2019 outcomes in men with HIV
- PMID: 35848570
- PMCID: PMC9444875
- DOI: 10.1097/QAD.0000000000003314
Tenofovir disoproxil fumarate and coronavirus disease 2019 outcomes in men with HIV
Abstract
Objective: To compare the risk of coronavirus disease 2019 (COVID-19) outcomes by antiretroviral therapy (ART) regimens among men with HIV.
Design: We included men with HIV on ART in the Veterans Aging Cohort Study who, between February 2020 and October 2021, were 18 years or older and had adequate virological control, CD4 + cell count, and HIV viral load measured in the previous 12 months, and no previous COVID-19 diagnosis or vaccination.
Methods: We compared the adjusted risks of documented severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, COVID-19-related hospitalization, and intensive care unit (ICU) admission by baseline ART regimen: tenofovir alafenamide (TAF)/emtricitabine (FTC), tenofovir disoproxil fumarate (TDF)/FTC, abacavir (ABC)/lamivudine (3TC), and other. We fit pooled logistic regressions to estimate the 18-month risks standardized by demographic and clinical factors.
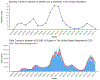
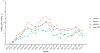
Results: Among 20 494 eligible individuals, the baseline characteristics were similar across regimens, except that TDF/FTC and TAF/FTC had lower prevalences of chronic kidney disease and estimated glomerular filtration rate <60 ml/min. Compared with TAF/FTC, the estimated 18-month risk ratio (95% confidence interval) of documented SARS-CoV-2 infection was 0.65 (0.43, 0.89) for TDF/FTC, 1.00 (0.85, 1.18) for ABC/3TC, and 0.87 (0.70, 1.04) for others. The corresponding risk ratios for COVID-19 hospitalization were 0.43 (0.07, 0.87), 1.09 (0.79, 1.48), and 1.21 (0.88, 1.62). The risk of COVID-19 ICU admission was lowest for TDF/FTC, but the estimates were imprecise.
Conclusion: Our study suggests that, in men living with HIV, TDF/FTC may protect against COVID-19-related events. Randomized trials are needed to investigate the effectiveness of TDF as prophylaxis for, and early treatment of, COVID-19 in the general population.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Figures

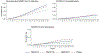
References
-
- World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. - PubMed
-
- Del Amo J, Polo R, Moreno S, Martínez E, Cabello A, Iribarren J, et al. Tenofovir Disoproxil Fumarate and severity of COVID-19 in people with HIV infection [preprint] medRxiv 2021:2021.2011.2011..
-
- Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2021; 73(7):e2005–e2015. - PMC - PubMed
-
- Munoz B, Buti M, Vazquez I, Conde M, Monterde V, Diaz F, et al. Tenofovir reduces the severity of COVID-19 infection in chronic hepatitis B patients [abstract]. Journal of Hepatology 2021:S746–S747.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

