Kir5.1 channels: potential role in epilepsy and seizure disorders
- PMID: 35848616
- PMCID: PMC9448276
- DOI: 10.1152/ajpcell.00235.2022
Kir5.1 channels: potential role in epilepsy and seizure disorders
Abstract
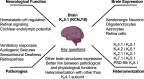
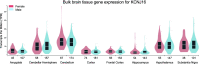
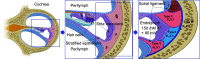
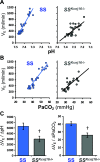
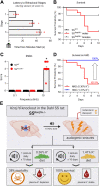
Inwardly rectifying potassium (Kir) channels are broadly expressed in many mammalian organ systems, where they contribute to critical physiological functions. However, the importance and function of the Kir5.1 channel (encoded by the KCNJ16 gene) have not been fully recognized. This review focuses on the recent advances in understanding the expression patterns and functional roles of Kir5.1 channels in fundamental physiological systems vital to potassium homeostasis and neurological disorders. Recent studies have described the role of Kir5.1-forming Kir channels in mouse and rat lines with mutations in the Kcnj16 gene. The animal research reveals distinct renal and neurological phenotypes, including pH and electrolyte imbalances, blunted ventilatory responses to hypercapnia/hypoxia, and seizure disorders. Furthermore, it was confirmed that these phenotypes are reminiscent of those in patient cohorts in which mutations in the KCNJ16 gene have also been identified, further suggesting a critical role for Kir5.1 channels in homeostatic/neural systems health and disease. Future studies that focus on the many functional roles of these channels, expanded genetic screening in human patients, and the development of selective small-molecule inhibitors for Kir5.1 channels, will continue to increase our understanding of this unique Kir channel family member.
Keywords: SIDS; deafness; kcnj10; kcnj16.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection "Inward Rectifying K+ Channels." Jerod Denton, PhD, and Eric Delpire, PhD, served as Guest Editors of this collection.
Figures



Similar articles
-
Expression, localization, and functional properties of inwardly rectifying K+ channels in the kidney.Am J Physiol Renal Physiol. 2020 Feb 1;318(2):F332-F337. doi: 10.1152/ajprenal.00523.2019. Epub 2019 Dec 16. Am J Physiol Renal Physiol. 2020. PMID: 31841387 Free PMC article. Review.
-
Genetic mutation of Kcnj16 identifies Kir5.1-containing channels as key regulators of acute and chronic pH homeostasis.FASEB J. 2019 Apr;33(4):5067-5075. doi: 10.1096/fj.201802257R. Epub 2019 Jan 3. FASEB J. 2019. PMID: 30605394 Free PMC article.
-
Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome.Proc Natl Acad Sci U S A. 2011 Jun 21;108(25):10361-6. doi: 10.1073/pnas.1101400108. Epub 2011 Jun 1. Proc Natl Acad Sci U S A. 2011. PMID: 21633011 Free PMC article.
-
Loss of transcriptional activation of the potassium channel Kir5.1 by HNF1β drives autosomal dominant tubulointerstitial kidney disease.Kidney Int. 2017 Nov;92(5):1145-1156. doi: 10.1016/j.kint.2017.03.034. Epub 2017 May 31. Kidney Int. 2017. PMID: 28577853 Free PMC article.
-
Inwardly rectifying potassium channel 5.1: Structure, function, and possible roles in diseases.Genes Dis. 2020 Mar 21;8(3):272-278. doi: 10.1016/j.gendis.2020.03.006. eCollection 2021 May. Genes Dis. 2020. PMID: 33997174 Free PMC article. Review.
Cited by
-
Special collection on inward rectifying K+ channels.Am J Physiol Cell Physiol. 2023 Mar 1;324(3):C603-C605. doi: 10.1152/ajpcell.00457.2022. Epub 2023 Jan 23. Am J Physiol Cell Physiol. 2023. PMID: 36689674 Free PMC article.
-
Relationship between right-to-left shunt, hypoxia, and epilepsy.Epilepsia Open. 2023 Jun;8(2):456-465. doi: 10.1002/epi4.12710. Epub 2023 Feb 27. Epilepsia Open. 2023. PMID: 36808903 Free PMC article.
-
Diverse functions of the inward-rectifying potassium channel Kir5.1 and its relationship with human diseases.Front Physiol. 2023 Feb 27;14:1127893. doi: 10.3389/fphys.2023.1127893. eCollection 2023. Front Physiol. 2023. PMID: 36923292 Free PMC article. Review.
-
Rodent Models of Audiogenic Epilepsy: Genetic Aspects, Advantages, Current Problems and Perspectives.Biomedicines. 2022 Nov 15;10(11):2934. doi: 10.3390/biomedicines10112934. Biomedicines. 2022. PMID: 36428502 Free PMC article. Review.
-
Multisite and multitimepoint proteomics reveal that patent foramen ovale closure improves migraine and epilepsy by reducing right-to-left shunt-induced hypoxia.MedComm (2020). 2023 Aug 12;4(4):e334. doi: 10.1002/mco2.334. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37576864 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

