Effect of baricitinib in regulating programmed death 1 and ligand programmed cell death ligand 1 through JAK/STAT pathway in psoriasis
- PMID: 35848689
- PMCID: PMC9396682
- DOI: 10.4103/ijp.ijp_1089_20
Effect of baricitinib in regulating programmed death 1 and ligand programmed cell death ligand 1 through JAK/STAT pathway in psoriasis
Abstract
Objectives: Psoriasis is a chronic infectious skin disease triggered by an autoimmune process involving T-cell-mediated hyper-proliferation of keratinocytes. The objective of this study is to assess the modulation of programmed death 1 (PD-1) and its ligand programmed cell death ligand 1 (PD-L1) through JAK/STAT pathway during the development of a psoriasis-like disease by both in vitro and in vivo model. Baricitinib, a known inhibitor of JAK1 and JAK2, was used to study the impact on PD-1 and PD-L1.
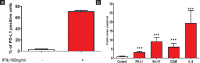
Materials and methods: Human peripheral blood mononuclear cells (PBMC) were stimulated with either anti-CD3/CD28 or PMA/Ionomycin, to modulate level of PD-1 and PD-L1 under psoriasis-like condition. Interferon-gamma (IFNγ) was used to treat HaCaT cells to mimic the diseased keratinocytes found in Psoriatic patients. Psoriasis was induced with Imiquimod (IMQ) in animal model to study the cross-talk between different cell types and pathways.
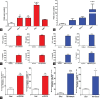
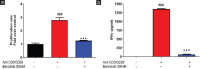
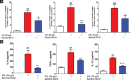
Results: Expression levels of PD-1 and PD-L1 in PBMC, and secretion of cytokines, namely tumor necrosis factor-α (TNFα), IFNγ, interleukin (IL)-6, and IL-1 β, were down-regulated on treatment with baricitinib. Further, in IFNγ-treated HaCaT cells (keratinocytes) mRNA levels of KRT-17 and PD-L1 were up-regulated.). Interestingly, in IFNγ-treated HaCat cells baricitinib decreased the levels of inflammatory cytokines such as IL-1 β, IL-6, and TNFα along with KRT-17 and PD-L1. On IFNγ-treatment. Data from both PBMC and HaCaT suggest an anti-inflammatory role for this compound. Accordingly, baricitinib was able to alleviate disease symptom in IMQ induce mice model of psoriasis. As a consequence of baricitinib treatment down-regulation of p-STAT3, PD- and PD-L1 expression levels were observed.
Conclusion: This study demonstrates a crosstalk between JAK/STAT and PD-1/PD-L1 pathways. It also demonstrates that cytokines such as IFNγ and IL-17 are down-regulated by baricitinib. We believe decreased expressions of PD-1 and PD-L1 may be a consequence of baricitinib-induced down-regulation of IFNγ and IL-17. More importantly, our data from the acute model of psoriasis indicates that PD-L1 behaves as a T-cell-associated T-cell-associated surrogate activation marker rather than immunosuppressive marker in early phase of psoriasis. Therefore it does not exhibit a causal relationship to disease.
Keywords: Baricitinib; HaCaT; imiquimod; programmed death 1; psoriasis.
Conflict of interest statement
None
Figures

Similar articles
-
Programmed cell death ligand 1 alleviates psoriatic inflammation by suppressing IL-17A production from programmed cell death 1-high T cells.J Allergy Clin Immunol. 2016 May;137(5):1466-1476.e3. doi: 10.1016/j.jaci.2015.11.021. Epub 2016 Jan 27. J Allergy Clin Immunol. 2016. PMID: 26824999
-
The JAK inhibitor baricitinib inhibits oncostatin M induction of proinflammatory mediators in ex-vivo synovial derived cells.Clin Exp Rheumatol. 2022 Sep;40(9):1620-1628. doi: 10.55563/clinexprheumatol/cfsajk. Epub 2021 Oct 13. Clin Exp Rheumatol. 2022. PMID: 34665696
-
Kaempferol modulates IFN-γ induced JAK-STAT signaling pathway and ameliorates imiquimod-induced psoriasis-like skin lesions.Int Immunopharmacol. 2023 Jan;114:109585. doi: 10.1016/j.intimp.2022.109585. Epub 2022 Dec 15. Int Immunopharmacol. 2023. PMID: 36527884
-
Preclinical development and clinical studies of targeted JAK/STAT combined Anti-PD-1/PD-L1 therapy.Int Immunopharmacol. 2024 Mar 30;130:111717. doi: 10.1016/j.intimp.2024.111717. Epub 2024 Feb 21. Int Immunopharmacol. 2024. PMID: 38387193 Review.
-
Clinical Implications of Targeting the JAK-STAT Pathway in Psoriatic Disease: Emphasis on the TYK2 Pathway.J Cutan Med Surg. 2023 Jan-Feb;27(1_suppl):3S-24S. doi: 10.1177/12034754221141680. Epub 2022 Dec 15. J Cutan Med Surg. 2023. PMID: 36519621 Review.
Cited by
-
The role of inflammation in central serous chorioretinopathy: From mechanisms to therapeutic prospects.Front Pharmacol. 2024 May 21;15:1200492. doi: 10.3389/fphar.2024.1200492. eCollection 2024. Front Pharmacol. 2024. PMID: 38835666 Free PMC article. Review.
-
Potential Mechanism of Fatigue Induction and Its Management by JAK Inhibitors in Inflammatory Rheumatic Diseases.J Inflamm Res. 2023 Sep 8;16:3949-3965. doi: 10.2147/JIR.S414739. eCollection 2023. J Inflamm Res. 2023. PMID: 37706062 Free PMC article. Review.
-
Mirroring UC care pathways in refractory immune checkpoint inhibitor (ICI)-mediated colitis: distinct features and common pathways.Clin J Gastroenterol. 2023 Oct;16(5):680-684. doi: 10.1007/s12328-023-01826-6. Epub 2023 Jul 15. Clin J Gastroenterol. 2023. PMID: 37452993
-
Local and Sustained Baricitinib Delivery to the Skin through Injectable Hydrogels Containing Reversible Thioimidate Adducts.Adv Healthc Mater. 2024 May;13(12):e2303256. doi: 10.1002/adhm.202303256. Epub 2024 Jan 26. Adv Healthc Mater. 2024. PMID: 38207170 Free PMC article.
References
-
- Kim DS, Je JH, Kim SH, Shin D, Kim TG, Kim DY, et al. Programmed death-ligand 1, 2 expressions are decreased in the psoriatic epidermis. Arch Dermatol Res. 2015;307:531–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

