Pro-apoptotic, anti-metastatic, and anti-telomerase activity of Tinospora cordifolia and its active polysaccharide arabinogalactan during Benzo(a)pyrene-induced lung carcinogenesis
- PMID: 35848691
- PMCID: PMC9396687
- DOI: 10.4103/ijp.ijp_962_20
Pro-apoptotic, anti-metastatic, and anti-telomerase activity of Tinospora cordifolia and its active polysaccharide arabinogalactan during Benzo(a)pyrene-induced lung carcinogenesis
Abstract
Background: The present study aims to unravel the pro-apoptotic, anti-metastatic, and anti-telomerase activity of aqueous extract of Tinospora cordifolia stem (Aq.Tc) and its active component arabinogalactan (AG) during Benzo(a)pyrene [B(a)P]-induced lung tumorigenesis in mice.
Materials and methods: Lung tumors were induced in male BALB/c mice using B(a)P as a carcinogen. Animals were administered twice with 50 mg/kg b.wt (i.p.) dosage of B(a)P at the 2nd and 4th week of the study. Mice were orally treated with Aq.Tc and AG on alternate days at a dose of 200 mg/kg b.wt and 7.5 mg/kg b.wt, respectively, for continuous 22 weeks.
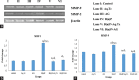
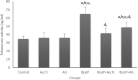
Results: Oral administration of animals with Aq.Tc and AG suppressed the development of lung carcinogenesis by modulating the mRNA and protein expressions of different apoptotic genes; bcl-2, bax, caspase 3, and caspase 9. The pro-apoptotic proficiency of Aq.Tc and AG was further confirmed by DNA agarose gel electrophoresis showing fragmentation in B(a)P + Aq.Tc group and smear formation in B(a)P + AG group. In contrast to the control group, an increase in tumor invasion factors such as matrix metalloproteinases-2 (MMP-2) and MMP-9 was also observed in B(a)P treated animals. Nevertheless, Aq.Tc and AG treatment effectively mitigated the B(a)P-induced upregulation of MMP-2 and MMP-9. The activity of the telomerase enzyme was also observed to be upregulated in B(a)P treated animals which consecutively found to get normalized with the parallel administration of Aq.Tc and AG.
Conclusion: Aq.Tc and AG successfully mitigated the altered expression of apoptosis, metastasis, and telomerase activity-associated genes during pulmonary carcinogenesis.
Keywords: Apoptosis; Tinospora cordifolia; lung cancer; metastasis; telomerase.
Conflict of interest statement
None
Figures




Similar articles
-
Tinospora cordifolia and arabinogalactan exert chemopreventive action during benzo(a)pyrene-induced pulmonary carcinogenesis: studies on ultrastructural, molecular, and biochemical alterations.Eur J Cancer Prev. 2021 Jan;30(1):21-39. doi: 10.1097/CEJ.0000000000000595. Eur J Cancer Prev. 2021. Retraction in: Eur J Cancer Prev. 2021 May 1;30(3):291. doi: 10.1097/CEJ.0000000000000665. PMID: 33122541 Retracted.
-
Tinospora Cordifolia and Arabinogalactan in combination modulates benzo(a)pyrene-induced genotoxicity during lung carcinogenesis.Drug Chem Toxicol. 2022 May;45(3):1427-1431. doi: 10.1080/01480545.2021.1995406. Epub 2021 Oct 28. Drug Chem Toxicol. 2022. PMID: 34711124
-
Chemopreventive role of arabinogalactan against experimentally induced pulmonary carcinogenesis: a study in relation to its initiation phase.Drug Chem Toxicol. 2021 Nov;44(6):642-654. doi: 10.1080/01480545.2019.1643877. Epub 2019 Aug 5. Drug Chem Toxicol. 2021. PMID: 31379226
-
Tinospora cordifolia (Willd.) Hook.f. & Thomson polysaccharides: A review on extraction, characterization, and bioactivities.Int J Biol Macromol. 2023 Feb 28;229:463-475. doi: 10.1016/j.ijbiomac.2022.12.181. Epub 2022 Dec 20. Int J Biol Macromol. 2023. PMID: 36563821 Review.
-
Potential use of Tinospora cordifolia as a herbal supplement in dairy animals: a review.Trop Anim Health Prod. 2022 Dec 11;55(1):4. doi: 10.1007/s11250-022-03415-0. Trop Anim Health Prod. 2022. PMID: 36502455 Review.
Cited by
-
Effect of Ayurveda and Siddha interventions in the management of chronic lymphocytic leukemia: A case report.J Ayurveda Integr Med. 2025 Jan-Feb;16(1):101017. doi: 10.1016/j.jaim.2024.101017. Epub 2024 Dec 14. J Ayurveda Integr Med. 2025. PMID: 39673829 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

