Antidepressants for social anxiety disorder: A systematic review and meta-analysis
- PMID: 35848723
- PMCID: PMC9773641
- DOI: 10.1002/npr2.12275
Antidepressants for social anxiety disorder: A systematic review and meta-analysis
Abstract
Aim: This systematic review is aimed to update and reintegrate the pharmacotherapy of social anxiety disorder (SAD), including the Japanese medical database.
Methods: We conducted a systematic review and meta-analysis of pharmacotherapy of SAD according to the Medical Information Distribution Service. We used data from a most recent systematic review, and updated search were conducted using MEDLINE, PubMed, CENTRAL, ICTRP, and ICHUSHI from August 1st, 2017 to January 31st, 2022. The outcome were response rates assessed by Clinical Global Impressions Improvement, efficacy assessed by the Liebowitz Social Anxiety Scale (LSAS), and dropout rates. We performed a random effect of meta-analysis to obtain the differences in each outcome between active medication and placebo. We used RevMan version 5.3 for analyses.
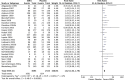
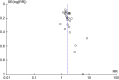
Results: We identified 5 studies through update search and performed meta-analysis for 33 studies on selective serotonin reuptake inhibitor (SSRI) and 6 studies on serotonin noradrenalin reuptake inhibitor (SNRI). The response rate (RR = 1.62) and the LSAS score reduction (mean difference = -9.65) of SSRI, and the response rate (RR = 1.57) and the LSAS score reduction (mean difference = -11.72) of SNRI were significantly different from placebo. The dropout rates of SSRI or SNRI were not significant. The response rates of SSRIs in both Japanese studies (RR = 1.44) and countries other than Japan (RR = 1.67) were significant. Most findings were based on low quality of evidence.
Conclusion: SSRIs are valid option for pharmacotherapy of SAD including Japanese patients. SNRIs are another effective option. However, the results should be interpreted cautiously due to several risk of bias.
Keywords: Japan; antidepressants; meta-analysis; social anxiety disorder; systematic review.
© 2022 The Authors. Neuropsychopharmacology Reports published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Society of Neuropsychopharmacology.
Conflict of interest statement
The authors have declared that there are no conflicts of interest in relation to the subject of this study. NM has received grant from Okamoto mental health foundation. SA received honoraria from Tanabe Mitsubishi Pharma, Mochida Pharmaceutical, Yoshitomiyakuhin and Shionogi & Co., LTD. HI reports lecture fees from Mochida Pharmaceutical and Otsuka Pharmaceutical, personal fees from Mitsubishi‐Tanabe pharma, personal fees from Kyowa pharmaceutical, outside the submitted work. Takeshi Inoue has received personal fees from Mochida Pharmaceutical, Takeda Pharmaceutical, Eli Lilly, Janssen Pharmaceutical, MSD, Taisho Toyama Pharmaceutical, Yoshitomiyakuhin, and Daiichi Sankyo; grants from Shionogi, Astellas, Tsumura, and Eisai; and grants and personal fees from Otsuka Pharmaceutical, Dainippon Sumitomo Pharma, Mitsubishi Tanabe Pharma, Kyowa Pharmaceutical Industry, Pfizer, Novartis Pharma, and Meiji Seika Pharma; and is a member of the advisory boards of Pfizer, Novartis Pharma, and Mitsubishi Tanabe Pharma. ES reports a joint research fund under contract from Sumitomo Dainippon Pharma, outside the submitted work.
Figures




References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc; 2013.
-
- Kawakami N, Takeshima T, Ono Y, Uda H, Hata Y, Nakane Y, et al. Twelve‐month prevalence, severity, and treatment of common mental disorders in communities in Japan: preliminary finding from the world mental health Japan survey 2002–2003. Psychiatry Clin Neurosci. 2005;59:441–52. - PubMed
-
- Wancata J, Fridl M, Friedrich F. Social phobia: epidemiology and health care. Psychiatr Danub. 2009;21:520–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

