Mathematical relationships between spinal motoneuron properties
- PMID: 35848819
- PMCID: PMC9612914
- DOI: 10.7554/eLife.76489
Mathematical relationships between spinal motoneuron properties
Abstract
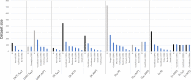
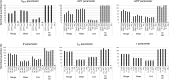
Our understanding of the behaviour of spinal alpha-motoneurons (MNs) in mammals partly relies on our knowledge of the relationships between MN membrane properties, such as MN size, resistance, rheobase, capacitance, time constant, axonal conduction velocity, and afterhyperpolarization duration. We reprocessed the data from 40 experimental studies in adult cat, rat, and mouse MN preparations to empirically derive a set of quantitative mathematical relationships between these MN electrophysiological and anatomical properties. This validated mathematical framework, which supports past findings that the MN membrane properties are all related to each other and clarifies the nature of their associations, is besides consistent with the Henneman's size principle and Rall's cable theory. The derived mathematical relationships provide a convenient tool for neuroscientists and experimenters to complete experimental datasets, explore the relationships between pairs of MN properties never concurrently observed in previous experiments, or investigate inter-mammalian-species variations in MN membrane properties. Using this mathematical framework, modellers can build profiles of inter-consistent MN-specific properties to scale pools of MN models, with consequences on the accuracy and the interpretability of the simulations.
Keywords: Henneman's size principle; mathematical relationships; motoneuron; motor neuron; motor neuron size; motor unit; neuroscience; none; physics of living systems.
Plain language summary
Muscles receive their instructions through electrical signals carried by tens or hundreds of cells connected to the command centers of the body. These ‘alpha-motoneurons’ have various sizes and electrical characteristics which affect how they transmit signals. Previous experiments have shown that these properties are linked; for instance, larger motoneurons transfer electrical signals more quickly. The exact nature of the mathematical relationships between these characteristics, however, remains unclear. This limits our understanding of the behaviour of motoneurons from experimental data. To identify the equations linking eight motoneuron properties, Caillet et al. analysed published datasets from experimental studies on cat motoneurons. This approach uncovered simple mathematical associations: in fact, only one characteristic needs to be measured experimentally to calculate all the other properties. The relationships identified were also consistent with previously accepted approaches for modelling motoneuron activity. Caillet et al. then validated this mathematical framework with data from studies on rodents, showing that some of the equations hold true for different mammals. This work offers a quick and easy way for researchers to calculate the characteristics of a motoneuron based on a single observation. This will allow non-measured properties to be added to experimental datasets, and it could help to uncover the diversity of motoneurons at work within a population.
© 2022, Caillet et al.
Conflict of interest statement
AC, AP, DF, LM No competing interests declared
Figures
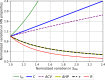
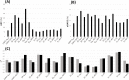

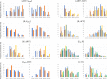
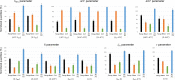
References
-
- Ankit R. WebPlotDigitizer. 4.4. 2020 https://automeris.io/WebPlotDigitizer
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

