Polarity switching of ovarian cancer cell clusters via SRC family kinase is involved in the peritoneal dissemination
- PMID: 35848881
- PMCID: PMC9530866
- DOI: 10.1111/cas.15493
Polarity switching of ovarian cancer cell clusters via SRC family kinase is involved in the peritoneal dissemination
Abstract
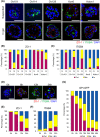
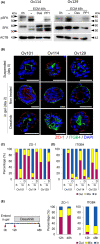
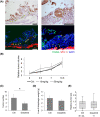
Peritoneal dissemination is a predominant pattern of metastasis in patients with advanced ovarian cancer. Despite recent progress in the management strategy, peritoneal dissemination remains a determinant of poor ovarian cancer prognosis. Using various histological types of patient-derived ovarian cancer organoids, the roles of the apicobasal polarity of ovarian cancer cell clusters in peritoneal dissemination were studied. First, it was found that both ovarian cancer tissues and ovarian organoids showed apicobasal polarity, where zonula occludens-1 (ZO-1) and integrin beta 4 (ITGB4) served as markers for apical and basal sides, respectively. The organoids in suspension culture, as a model of cancer cell cluster floating in ascites, showed apical-out/basal-in polarity status, while once embedded in extracellular matrix (ECM), the organoids switched their polarity to apical-in/basal-out. This polarity switch was accompanied by the SRC kinase family (SFK) phosphorylation and was inhibited by SFK inhibitors. SFK inhibitors abrogated the adherence of the organoids onto the ECM-coated plastic surface. When the organoids were seeded on a mesothelial cell layer, they cleared and invaded mesothelial cells. In vivo, dasatinib, an SFK inhibitor, suppressed peritoneal dissemination of ovarian cancer organoids in immunodeficient mice. These results suggest SFK-mediated polarity switching is involved in peritoneal metastasis. Polarity switching would be a potential therapeutic target for suppressing peritoneal dissemination in ovarian cancer.
Keywords: SRC family kinase; cell polarity; metastasis; organoids; ovarian cancer.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



References
-
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280‐304. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

