Mechanisms of resistance to immune checkpoint inhibitors
- PMID: 35848888
- PMCID: PMC9530865
- DOI: 10.1111/cas.15497
Mechanisms of resistance to immune checkpoint inhibitors
Abstract
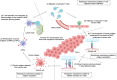
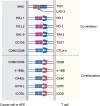
Immune checkpoint inhibitors (ICIs) are effective for various types of cancer, and their application has led to paradigm shifts in cancer treatment. While many patients can obtain clinical benefits from ICI treatment, a large number of patients are primarily resistant to such treatment or acquire resistance after an initial response. Thus, elucidating the resistance mechanisms is warranted to improve the clinical outcomes of ICI treatment. ICIs exert their antitumor effects by activating T cells in the tumor microenvironment. There are various resistance mechanisms, such as insufficient antigen recognition by T cells, impaired T-cell migration and/or infiltration, and reduced T-cell cytotoxicity, most of which are related to the T-cell activation process. Thus, we classify them into three main mechanisms: resistance mechanisms related to antigen recognition, T-cell migration and/or infiltration, and effector functions of T cells. In this review, we summarize these mechanisms of resistance to ICIs related to the T-cell activation process and progress in the development of novel therapies that can overcome resistance.
Keywords: T cell; cancer immunology; exhaustion; immune checkpoint inhibitor; resistance.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Y. T. is a current editorial board member of Cancer Science. Y. T. received research grants and honoraria from Ono Pharmaceutical and Bristol‐Myers Squibb; research grants from KOTAI Biotechnologies, Daiichi‐Sankyo, and KORTUC; and honoraria from Chugai Pharmaceutical and MSD outside this work.
Figures

References
-
- Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. - PubMed
-
- Hellmann MD, Paz‐Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non‐small‐cell lung cancer. N Engl J Med. 2019;381:2020‐2031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 19ck0106521h0001/Japan Agency for Medical Research and Development
- 22ama221303h0001/Japan Agency for Medical Research and Development
- 21-211033868/Japan Science and Technology Agency
- Grants-in-Aid for Scientific Research (B, 20H03694)/Japan Society for the Promotion of Science
- 21cm0106383/Project for Cancer Research and Therapeutic Evolution
LinkOut - more resources
Full Text Sources
Medical

