Limitations of microbial iron reduction under extreme conditions
- PMID: 35849069
- PMCID: PMC9629499
- DOI: 10.1093/femsre/fuac033
Limitations of microbial iron reduction under extreme conditions
Abstract
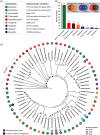
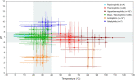
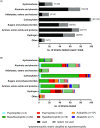
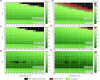
Microbial iron reduction is a widespread and ancient metabolism on Earth, and may plausibly support microbial life on Mars and beyond. Yet, the extreme limits of this metabolism are yet to be defined. To investigate this, we surveyed the recorded limits to microbial iron reduction in a wide range of characterized iron-reducing microorganisms (n = 141), with a focus on pH and temperature. We then calculated Gibbs free energy of common microbially mediated iron reduction reactions across the pH-temperature habitability space to identify thermodynamic limits. Comparing predicted and observed limits, we show that microbial iron reduction is generally reported at extremes of pH or temperature alone, but not when these extremes are combined (with the exception of a small number of acidophilic hyperthermophiles). These patterns leave thermodynamically favourable combinations of pH and temperature apparently unoccupied. The empty spaces could be explained by experimental bias, but they could also be explained by energetic and biochemical limits to iron reduction at combined extremes. Our data allow for a review of our current understanding of the limits to microbial iron reduction at extremes and provide a basis to test more general hypotheses about the extent to which biochemistry establishes the limits to life.
Keywords: biochemistry; extremophiles; limits to life; microbial iron reduction; thermodynamics.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures


References
-
- Boone DR, Liu Y, Zhao Z-J. et al. Bacillus infernus sp. nov., an Fe(III)- and Mn(IV)-Reducing Anaerobe from the Deep Terrestrial Subsurface. Int J Syst Evol Microbiol. 1995;45:441–8. - PubMed
-
- Canfield D E, Jørgensen BB, Fossing Het al. . Pathways of organic carbon oxidation in three continental margin sediments. Mar Geol. 1993;113:27–40. - PubMed
-
- Chen Y, He Y, Shao Zet al. . Thermosipho ferrireducens sp.nov., an anaerobic thermophilic iron(III)-reducing bacterium isolated from a deep-sea hydrothermal sulfide deposits. Int J Syst Evol Microbiol. 2021;71. DOI: 10.1099/ijsem.0.004929. - PubMed
-
- Coates JD, Bhupathiraju VK, Achenbach LAet al. . Geobacter hydrogenophilus, Geobacter chapelli and Geobacter grbiciae, three new, strictly anaerobic, dissimilatory Fe(III)-reducers. Int J Syst Evol Microbiol. 2001;51:581–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

