Population pharmacokinetics of apramycin from first-in-human plasma and urine data to support prediction of efficacious dose
- PMID: 35849148
- PMCID: PMC9525081
- DOI: 10.1093/jac/dkac225
Population pharmacokinetics of apramycin from first-in-human plasma and urine data to support prediction of efficacious dose
Abstract
Background: Apramycin is under development for human use as EBL-1003, a crystalline free base of apramycin, in face of increasing incidence of multidrug-resistant bacteria. Both toxicity and cross-resistance, commonly seen for other aminoglycosides, appear relatively low owing to its distinct chemical structure.
Objectives: To perform a population pharmacokinetic (PPK) analysis and predict an efficacious dose based on data from a first-in-human Phase I trial.
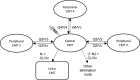
Methods: The drug was administered intravenously over 30 min in five ascending-dose groups ranging from 0.3 to 30 mg/kg. Plasma and urine samples were collected from 30 healthy volunteers. PPK model development was performed stepwise and the final model was used for PTA analysis.
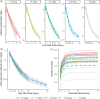
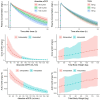
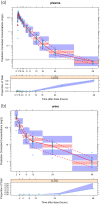
Results: A mammillary four-compartment PPK model, with linear elimination and a renal fractional excretion of 90%, described the data. Apramycin clearance was proportional to the absolute estimated glomerular filtration rate (eGFR). All fixed effect parameters were allometrically scaled to total body weight (TBW). Clearance and steady-state volume of distribution were estimated to 5.5 L/h and 16 L, respectively, for a typical individual with absolute eGFR of 124 mL/min and TBW of 70 kg. PTA analyses demonstrated that the anticipated efficacious dose (30 mg/kg daily, 30 min intravenous infusion) reaches a probability of 96.4% for a free AUC/MIC target of 40, given an MIC of 8 mg/L, in a virtual Phase II patient population with an absolute eGFR extrapolated to 80 mL/min.
Conclusions: The results support further Phase II clinical trials with apramycin at an anticipated efficacious dose of 30 mg/kg once daily.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

