Toxicity Profiles of Systemic Therapies for Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-analysis
- PMID: 35849393
- PMCID: PMC9295000
- DOI: 10.1001/jamanetworkopen.2022.22721
Toxicity Profiles of Systemic Therapies for Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-analysis
Abstract
Importance: The recent development of targeted therapy and immunotherapy has made neoadjuvant therapy an attractive option for patients with hepatocellular carcinoma (HCC). However, surgeons are concerned that adverse effects of neoadjuvant therapy with these agents could lead to delayed or even cancelled surgeries.
Objective: To summarize the current evidence regarding toxicity profiles for tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) among patients with HCC.
Data sources: Medline, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from January 1990 and December 2021.
Study selection: Single-group, placebo-controlled, and dual-agent clinical trials comparing TKIs and ICIs in patients with HCC were eligible for inclusion.
Data extraction and synthesis: Following the Preferred Reporting Items in Systematic Reviews and Meta-analysis guideline, 2 reviewers independently extracted data. A random-effects model was used.
Main outcomes and measures: The primary outcome was the proportion of patients with clinically significant liver-related adverse events. Secondary outcomes included the proportion of patients who experienced clinically relevant (grade 3 or higher) adverse events and significant adverse events (ie, those that were life threatening, required hospitalization, or prolonged disability) as well as the risk ratio (RR) of these complications.
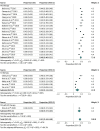
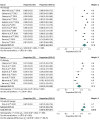
Results: Overall, 30 studies with 12 921 patients were included. Patients had a mean (range) age of 62 (18-89) years; a mean (SD) 84% (3) were male; a mean (SD) 82% (16) had Barcelona Clinic Liver Cancer stage C HCC; and a mean (SD) 97% (6) had Childs A cirrhosis. Overall, 21% (95% CI, 16%-26%) of patients receiving TKIs had liver toxic effects compared with 28% (95% CI, 21%-35%) of patients receiving ICIs. Severe adverse events occurred in 46% (95% CI, 40%-51%) of patients receiving TKIs compared with 24% (95% CI, 13%-35%) of patients receiving ICIs. Compared with patients receiving sorafenib, other TKIs were associated with similar rates of liver toxic effects (RR, 1.06; 95% CI, 0.92-1.24) but higher rates of severe adverse events (RR, 1.24; 95% CI, 1.07-1.44). Comparing ICIs with sorafenib, there were similar rates of liver toxic effects (RR, 1.10; 95% CI, 0.86-1.40) and severe adverse events (RR, 1.19; 95% CI, 0.95-1.50).
Conclusions and relevance: In this systematic review and meta-analysis, serious adverse events were lower with ICIs than with TKIs, while liver toxic effects were similar. Combination therapy with novel ICIs is an appealing option in trials of neoadjuvant therapy for patients with HCC, requiring evaluation in preoperative trials.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

