Membrane curvature and PS localize coagulation proteins to filopodia and retraction fibers of endothelial cells
- PMID: 35849711
- PMCID: PMC9827038
- DOI: 10.1182/bloodadvances.2021006870
Membrane curvature and PS localize coagulation proteins to filopodia and retraction fibers of endothelial cells
Abstract
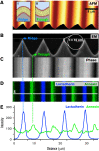
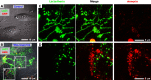
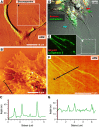
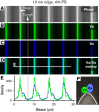
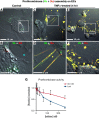
Prior reports indicate that the convex membrane curvature of phosphatidylserine (PS)-containing vesicles enhances formation of binding sites for factor Va and lactadherin. Yet, the relationship of convex curvature to localization of these proteins on cells remains unknown. We developed a membrane topology model, using phospholipid bilayers supported by nano-etched silica substrates, to further explore the relationship between curvature and localization of coagulation proteins. Ridge convexity corresponded to maximal curvature of physiologic membranes (radii of 10 or 30 nm) and the troughs had a variable concave curvature. The benchmark PS probe lactadherin exhibited strong differential binding to the ridges, on membranes with 4% to 15% PS. Factor Va, with a PS-binding motif homologous to lactadherin, also bound selectively to the ridges. Bound factor Va supported coincident binding of factor Xa, localizing prothrombinase complexes to the ridges. Endothelial cells responded to prothrombotic stressors and stimuli (staurosporine, tumor necrosis factor-α [TNF- α]) by retracting cell margins and forming filaments and filopodia. These had a high positive curvature similar to supported membrane ridges and selectively bound lactadherin. Likewise, the retraction filaments and filopodia bound factor Va and supported assembly of prothrombinase, whereas the cell body did not. The perfusion of plasma over TNF-α-stimulated endothelia in culture dishes and engineered 3-dimensional microvessels led to fibrin deposition at cell margins, inhibited by lactadherin, without clotting of bulk plasma. Our results indicate that stressed or stimulated endothelial cells support prothrombinase activity localized to convex topological features at cell margins. These findings may relate to perivascular fibrin deposition in sepsis and inflammation.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Similar articles
-
Trp2063 and Trp2064 in the factor Va C2 domain are required for high-affinity binding to phospholipid membranes but not for assembly of the prothrombinase complex.Biochemistry. 2004 Apr 13;43(14):4385-93. doi: 10.1021/bi035763o. Biochemistry. 2004. PMID: 15065883
-
Prothrombin contributes to the assembly of the factor Va-factor Xa complex at phosphatidylserine-containing phospholipid membranes.J Biol Chem. 1995 Nov 10;270(45):26883-9. doi: 10.1074/jbc.270.45.26883. J Biol Chem. 1995. PMID: 7592932
-
Thrombotic Role of Blood and Endothelial Cells in Uremia through Phosphatidylserine Exposure and Microparticle Release.PLoS One. 2015 Nov 16;10(11):e0142835. doi: 10.1371/journal.pone.0142835. eCollection 2015. PLoS One. 2015. PMID: 26580207 Free PMC article.
-
Increased blood cell phosphatidylserine exposure and circulating microparticles contribute to procoagulant activity after carotid artery stenting.J Neurosurg. 2017 Nov;127(5):1041-1054. doi: 10.3171/2016.8.JNS16996. Epub 2016 Dec 23. J Neurosurg. 2017. PMID: 28009236
-
Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature.Biochim Biophys Acta. 2004 Nov 17;1667(1):82-90. doi: 10.1016/j.bbamem.2004.09.006. Biochim Biophys Acta. 2004. PMID: 15533308
Cited by
-
Engineered Fluorescent Variants of Lactadherin C2 Domain for Phosphatidylserine Detection in Flow Cytometry.Biomolecules. 2025 May 6;15(5):673. doi: 10.3390/biom15050673. Biomolecules. 2025. PMID: 40427566 Free PMC article.
-
Effect of endothelial responses on sepsis-associated organ dysfunction.Chin Med J (Engl). 2024 Dec 5;137(23):2782-2792. doi: 10.1097/CM9.0000000000003342. Epub 2024 Nov 6. Chin Med J (Engl). 2024. PMID: 39501810 Free PMC article. Review.
-
Extracellular vesicles in atherosclerosis cardiovascular disease: emerging roles and mechanisms.Front Cardiovasc Med. 2025 Jun 24;12:1611557. doi: 10.3389/fcvm.2025.1611557. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40630905 Free PMC article. Review.
-
4D intravital imaging studies identify platelets as the predominant cellular procoagulant surface in a mouse hemostasis model.Blood. 2024 Sep 5;144(10):1116-1126. doi: 10.1182/blood.2023022608. Blood. 2024. PMID: 38820498
References
-
- Shi J, Shi Y, Waehrens LN, Rasmussen JT, Heegaard CW, Gilbert GE. Lactadherin detects early phosphatidylserine exposure on immortalized leukemia cells undergoing programmed cell death. Cytometry A. 2006;69A(12):1193–1201. - PubMed
-
- Zhou J, Shi J, Hou J, et al. Phosphatidylserine exposure and procoagulant activity in acute promyelocytic leukemia. J Thromb Haemost. 2010;8(4):773–782. - PubMed
-
- van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114(Pt 20):3631–3642. - PubMed

