Venetoclax Plus Gilteritinib for FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia
- PMID: 35849791
- PMCID: PMC9746764
- DOI: 10.1200/JCO.22.00602
Venetoclax Plus Gilteritinib for FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia
Abstract
Purpose: The FMS-related tyrosine kinase 3 (FLT3) inhibitor gilteritinib is standard therapy for relapsed/refractory FLT3-mutated (FLT3mut) acute myeloid leukemia (AML) but seldom reduces FLT3mut burden or induces sustained efficacy. Gilteritinib combines synergistically with the BCL-2 inhibitor venetoclax in preclinical models of FLT3mut AML.
Methods: This phase Ib open-label, dose-escalation/dose-expansion study (ClinicalTrials.gov identifier: NCT03625505) enrolled patients with FLT3 wild-type and FLT3mut (escalation) or FLT3mut (expansion) relapsed/refractory AML. Patients received 400 mg oral venetoclax once daily and 80 mg or 120 mg oral gilteritinib once daily. The primary objectives were safety, identification of the recommended phase II dose, and the modified composite complete response (mCRc) rate (complete response [CR] + CR with incomplete blood count recovery + CR with incomplete platelet recovery + morphologic leukemia-free state) using ADMIRAL phase III-defined response criteria.
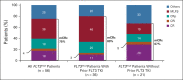
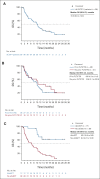
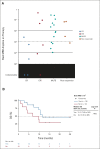
Results: Sixty-one patients were enrolled (n = 56 FLT3mut); 64% (n = 36 of 56) of FLT3mut patients had received prior FLT3 inhibitor therapy. The recommended phase II dose was 400 mg venetoclax once daily and 120 mg gilteritinib once daily. The most common grade 3/4 adverse events were cytopenias (n = 49; 80%). Adverse events prompted venetoclax and gilteritinib dose interruptions in 51% and 48%, respectively. The mCRc rate for FLT3mut patients was 75% (CR, 18%; CR with incomplete blood count recovery, 4%; CR with incomplete platelet recovery, 18%; and morphologic leukemia-free state, 36%) and was similar among patients with or without prior FLT3 inhibitor therapy (80% v 67%, respectively). The median follow-up was 17.5 months. The median time to response was 0.9 months, and the median remission duration was 4.9 months (95% CI, 3.4 to 6.6). FLT3 molecular response (< 10-2) was achieved in 60% of evaluable mCRc patients (n = 15 of 25). The median overall survival for FLT3mut patients was 10.0 months.
Conclusion: The combination of venetoclax and gilteritinib was associated with high mCRc and FLT3 molecular response rates regardless of prior FLT3 inhibitor exposure. Dose interruptions were needed to mitigate myelosuppression.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

Comment in
-
A New Dancing Partner for Venetoclax: Gilteritinib.J Clin Oncol. 2022 Dec 10;40(35):4033-4036. doi: 10.1200/JCO.22.01386. Epub 2022 Sep 2. J Clin Oncol. 2022. PMID: 36054870 No abstract available.
References
-
- Orlowski RJ, Mangan JK, Luger SM: Approach to patients with primary refractory acute myeloid leukemia. Curr Opin Hematol 22:97-107, 2015 - PubMed
-
- Roboz GJ, Rosenblat T, Arellano M, et al. : International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 32:1919-1926, 2014 - PubMed

