Estrogens rapidly shape synaptic and intrinsic properties to regulate the temporal precision of songbird auditory neurons
- PMID: 35849820
- PMCID: PMC10068288
- DOI: 10.1093/cercor/bhac280
Estrogens rapidly shape synaptic and intrinsic properties to regulate the temporal precision of songbird auditory neurons
Abstract
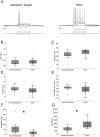
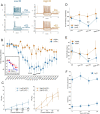
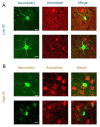
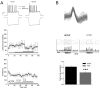
Sensory neurons parse millisecond-variant sound streams like birdsong and speech with exquisite precision. The auditory pallial cortex of vocal learners like humans and songbirds contains an unconventional neuromodulatory system: neuronal expression of the estrogen synthesis enzyme aromatase. Local forebrain neuroestrogens fluctuate when songbirds hear a song, and subsequently modulate bursting, gain, and temporal coding properties of auditory neurons. However, the way neuroestrogens shape intrinsic and synaptic properties of sensory neurons remains unknown. Here, using a combination of whole-cell patch clamp electrophysiology and calcium imaging, we investigate estrogenic neuromodulation of auditory neurons in a region resembling mammalian auditory association cortex. We found that estradiol rapidly enhances the temporal precision of neuronal firing via a membrane-bound G-protein coupled receptor and that estradiol rapidly suppresses inhibitory synaptic currents while sparing excitation. Notably, the rapid suppression of intrinsic excitability by estradiol was predicted by membrane input resistance and was observed in both males and females. These findings were corroborated by analysis of in vivo electrophysiology recordings, in which local estrogen synthesis blockade caused acute disruption of the temporal correlation of song-evoked firing patterns. Therefore, on a modulatory timescale, neuroestrogens alter intrinsic cellular properties and inhibitory neurotransmitter release to regulate the temporal precision of higher-order sensory neurons.
Keywords: aromatase; cortex; estradiol; learning; neuromodulation; zebra finch.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Aromatase and nonaromatase neurons in the zebra finch secondary auditory forebrain are indistinct in their song-driven gene induction and intrinsic electrophysiological properties.Eur J Neurosci. 2021 Nov;54(9):7072-7091. doi: 10.1111/ejn.15463. Epub 2021 Oct 7. Eur J Neurosci. 2021. PMID: 34535925 Free PMC article.
-
Neuroestrogen signaling in the songbird auditory cortex propagates into a sensorimotor network via an 'interface' nucleus.Neuroscience. 2015 Jan 22;284:522-535. doi: 10.1016/j.neuroscience.2014.10.023. Epub 2014 Oct 19. Neuroscience. 2015. PMID: 25453773 Free PMC article.
-
Norepinephrine Modulates Coding of Complex Vocalizations in the Songbird Auditory Cortex Independent of Local Neuroestrogen Synthesis.J Neurosci. 2015 Jun 24;35(25):9356-68. doi: 10.1523/JNEUROSCI.4445-14.2015. J Neurosci. 2015. PMID: 26109659 Free PMC article.
-
Neuroestrogens rapidly shape auditory circuits to support communication learning and perception: Evidence from songbirds.Horm Behav. 2018 Aug;104:77-87. doi: 10.1016/j.yhbeh.2018.03.007. Epub 2018 Mar 30. Horm Behav. 2018. PMID: 29555375 Free PMC article. Review.
-
Sex differences and rapid estrogen signaling: A look at songbird audition.Front Neuroendocrinol. 2015 Jul;38:37-49. doi: 10.1016/j.yfrne.2015.01.001. Epub 2015 Jan 28. Front Neuroendocrinol. 2015. PMID: 25637753 Free PMC article. Review.
Cited by
-
Rapid nongenomic estrogen signaling controls alcohol drinking behavior in mice.Nat Commun. 2024 Dec 30;15(1):10725. doi: 10.1038/s41467-024-54737-6. Nat Commun. 2024. PMID: 39737915 Free PMC article.
-
Estradiol decreases the excitability of RA projection neurons in adult male zebra finches.Front Cell Neurosci. 2023 Feb 14;17:1046984. doi: 10.3389/fncel.2023.1046984. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36866064 Free PMC article.
-
Acute Aromatase Inhibition Impairs Neural and Behavioral Auditory Scene Analysis in Zebra Finches.eNeuro. 2024 Mar 22;11(3):ENEURO.0423-23.2024. doi: 10.1523/ENEURO.0423-23.2024. Print 2024 Mar. eNeuro. 2024. PMID: 38467426 Free PMC article.
References
-
- Acharya KD, Veney SL. Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. 2012:72(11):1433–1446. - PubMed
-
- Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011:191:139–147. - PubMed
-
- Balthazart J, Ball G. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006:29(5):241–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

