Humoral and Cellular Immune Response After Third and Fourth SARS-CoV-2 mRNA Vaccination in Liver Transplant Recipients
- PMID: 35850415
- PMCID: PMC9287575
- DOI: 10.1016/j.cgh.2022.06.028
Humoral and Cellular Immune Response After Third and Fourth SARS-CoV-2 mRNA Vaccination in Liver Transplant Recipients
Abstract
Background & aims: Liver transplant recipients (LTRs) show a decreased immune response after 2 severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) vaccinations compared with healthy controls (HCs). Here, we investigated the immunogenicity of additional vaccinations.
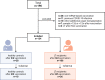
Methods: In this prospective study, humoral (anti-SARS-CoV-2 receptor-binding domain [anti-S RBD]) and cellular (interferon-gamma release assay) immune responses were determined after mRNA-based SARS-CoV-2 vaccination in 106 LTRs after a third vaccination and in 36 LTRs after a fourth vaccination. Patients with anti-S RBD antibody levels >0.8 arbitrary unit (AU)/mL after vaccination were defined as responders.
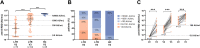
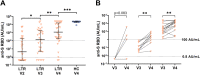
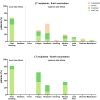
Results: After 3 vaccinations, 92% (97/106) of LTRs compared with 100% (28/28) of HCs were responders. However, the antibody titer of LTRs was lower compared with HCs (1891.0 vs 21,857.0 AU/mL; P < .001). Between a second and third vaccination (n = 75), the median antibody level increased 67-fold in LTRs. In patients seronegative after 2 vaccinations, a third dose induced seroconversion in 76% (19/25), whereas all HCs were already seropositive after 2 vaccinations. A spike-specific T-cell response was detected in 72% (28/39) after a third vaccination compared with 32% (11/34) after a second vaccination. Independent risk factors for a low antibody response (anti-S RBD <100 AU/mL) were first vaccination within the first year after liver transplant (odds ratio [OR], 8.00; P = .023), estimated glomular filtration rate <45 mL/min (OR, 4.72; P = .006), and low lymphocyte counts (OR, 5.02; P = .008). A fourth vaccination induced a 9-fold increase in the median antibody level and seroconversion in 60% (3/5) of previous non-responders.
Conclusions: A third and fourth SARS-CoV-2 vaccination effectively increases the humoral and cellular immune response of LTRs, but to a lesser extent than in HCs. A fourth vaccination should be generally considered in LTRs.
Keywords: COVID-19; Immunosuppression; Liver Transplant Recipients; T Cells; Third and Fourth SARS-CoV-2 Vaccination.
Copyright © 2022 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

