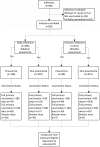
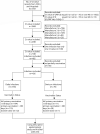
A case-case study on the effect of primary and booster immunization with China-produced COVID-19 vaccines on prevention of pneumonia and viral load among vaccinated persons infected by Delta and Omicron variants
- PMID: 35850623
- PMCID: PMC9359169
- DOI: 10.1080/22221751.2022.2103455
A case-case study on the effect of primary and booster immunization with China-produced COVID-19 vaccines on prevention of pneumonia and viral load among vaccinated persons infected by Delta and Omicron variants
Abstract
Using a three-prefecture, two-variant COVID-19 outbreak in Henan province in January 2022, we evaluated the associations of primary and booster immunization with China-produced COVID-19 vaccines and COVID-19 pneumonia and SARS-CoV-2 viral load among persons infected by Delta or Omicron variant. We obtained demographic, clinical, vaccination, and multiple Ct values of infections ≥3 years of age. Vaccination status was either primary series ≥180 days prior to infection; primary series <180 days prior to infection, or booster dose recipient. We used logistic regression to determine odds ratios (OR) of Delta and Omicron COVID-19 pneumonia by vaccination status. We analysed minimum Ct values by vaccination status, age, and variant. Of 826 eligible cases, 405 were Delta and 421 were Omicron cases; 48.9% of Delta and 19.0% of Omicron cases had COVID-19 pneumonia. Compared with full primary vaccination ≥180 days before infection, the aOR of pneumonia was 0.48 among those completing primary vaccination <180 days and 0.18 among booster recipients among these Delta infections. Among Omicron infections, the corresponding aOR was 0.34 among those completing primary vaccination <180 days. There were too few (ten) Omicron cases among booster dose recipients to calculate a reliable OR. There were no differences in minimum Ct values by vaccination status among the 356 Delta cases or 70 Omicron cases. COVID-19 pneumonia was less common among Omicron cases than Delta cases. Full primary vaccination reduced pneumonia effectively for 6 months; boosting six months after primary vaccination resulted in further reduction. We recommend accelerating the pace of booster dose administration.
Keywords: COVID-19 vaccine; Ct value; booster immunization; case-case study; real-world study.
Figures
Similar articles
-
Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants.JAMA. 2022 Feb 15;327(7):639-651. doi: 10.1001/jama.2022.0470. JAMA. 2022. PMID: 35060999 Free PMC article.
-
A Quick Displacement of the SARS-CoV-2 variant Delta with Omicron: Unprecedented Spike in COVID-19 Cases Associated with Fewer Admissions and Comparable Upper Respiratory Viral Loads.medRxiv [Preprint]. 2022 Jan 28:2022.01.26.22269927. doi: 10.1101/2022.01.26.22269927. medRxiv. 2022. Update in: EBioMedicine. 2022 May;79:104008. doi: 10.1016/j.ebiom.2022.104008. PMID: 35118480 Free PMC article. Updated. Preprint.
-
Relative effectiveness of booster vs. 2-dose mRNA Covid-19 vaccination in the Veterans Health Administration: Self-controlled risk interval analysis.Vaccine. 2022 Aug 5;40(33):4742-4747. doi: 10.1016/j.vaccine.2022.06.047. Epub 2022 Jun 21. Vaccine. 2022. PMID: 35773122 Free PMC article.
-
The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis.Front Public Health. 2022 Jul 13;10:940956. doi: 10.3389/fpubh.2022.940956. eCollection 2022. Front Public Health. 2022. PMID: 35910897 Free PMC article.
-
Duration of immunity to SARS-CoV-2 in children after natural infection or vaccination in the omicron and pre-omicron era: A systematic review of clinical and immunological studies.Front Immunol. 2023 Jan 11;13:1024924. doi: 10.3389/fimmu.2022.1024924. eCollection 2022. Front Immunol. 2023. PMID: 36713374 Free PMC article.
Cited by
-
Impact of Vaccination and Public Health Measures on the Severity of SARS-CoV-2 Omicron Infections in China: A Systematic Review and Meta-Regression Analysis.Vaccines (Basel). 2025 Jul 12;13(7):747. doi: 10.3390/vaccines13070747. Vaccines (Basel). 2025. PMID: 40733725 Free PMC article. Review.
-
Vaccinate with Confidence and Finish Strong.China CDC Wkly. 2022 Sep 16;4(37):828-831. doi: 10.46234/ccdcw2022.172. China CDC Wkly. 2022. PMID: 36284540 Free PMC article. No abstract available.
-
Effectiveness of inactivated COVID-19 vaccines against mild disease, pneumonia, and severe disease among persons infected with SARS-CoV-2 Omicron variant: real-world study in Jilin Province, China.Emerg Microbes Infect. 2023 Dec;12(1):2149935. doi: 10.1080/22221751.2022.2149935. Emerg Microbes Infect. 2023. PMID: 36398721 Free PMC article.
-
Effectiveness of Inactivated COVID-19 Vaccines against COVID-19 Caused by the SARS-CoV-2 Delta and Omicron Variants: A Retrospective Cohort Study.Vaccines (Basel). 2022 Oct 19;10(10):1753. doi: 10.3390/vaccines10101753. Vaccines (Basel). 2022. PMID: 36298618 Free PMC article.
-
Safety, Immunogenicity, and Effectiveness of Chinese-Made COVID-19 Vaccines in the Real World: An Interim Report of a Living Systematic Review.Vaccines (Basel). 2024 Jul 16;12(7):781. doi: 10.3390/vaccines12070781. Vaccines (Basel). 2024. PMID: 39066419 Free PMC article. Review.
References
-
- World Health Organization . Available from: https://covid19.who.int/.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous