Effect of epidural spinal cord stimulation after chronic spinal cord injury on volitional movement and cardiovascular function: study protocol for the phase II open label controlled E-STAND trial
- PMID: 35851008
- PMCID: PMC9297213
- DOI: 10.1136/bmjopen-2021-059126
Effect of epidural spinal cord stimulation after chronic spinal cord injury on volitional movement and cardiovascular function: study protocol for the phase II open label controlled E-STAND trial
Abstract
Introduction: Spinal cord injury (SCI) leads to significant changes in morbidity, mortality and quality of life (QOL). Currently, there are no effective therapies to restore function after chronic SCI. Preliminary studies have indicated that epidural spinal cord stimulation (eSCS) is a promising therapy to improve motor control and autonomic function for patients with chronic SCI. The aim of this study is to assess the effects of tonic eSCS after chronic SCI on quantitative outcomes of volitional movement and cardiovascular function. Our secondary objective is to optimise spinal cord stimulation parameters for volitional movement.
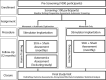
Methods and analysis: The Epidural Stimulation After Neurologic Damage (ESTAND) trial is a phase II single-site self-controlled trial of epidural stimulation with the goal of restoring volitional movement and autonomic function after motor complete SCI. Participants undergo epidural stimulator implantation and are followed up over 15 months while completing at-home, mobile application-based movement testing. The primary outcome measure integrates quantity of volitional movement and similarity to normal controls using the volitional response index (VRI) and the modified Brain Motor Control Assessment. The mobile application is a custom-designed platform to support participant response and a kinematic task to optimise the settings for each participant. The application optimises stimulation settings by evaluating the parameter space using movement data collected from the tablet application and accelerometers. A subgroup of participants with cardiovascular dysautonomia are included for optimisation of blood pressure stabilisation. Indirect effects of stimulation on cardiovascular function, pain, sexual function, bowel/bladder, QOL and psychiatric measures are analysed to assess generalisability of this targeted intervention.
Ethics and dissemination: This study has been approved after full review by the Minneapolis Medical Research Foundation Institutional Review Board and by the Minneapolis VA Health Care System. This project has received Food and Drug Administration investigational device exemption approval. Trial results will be disseminated through peer-reviewed publications, conference presentations and seminars.
Trial registration number: NCT03026816.
Keywords: neurological injury; neurosurgery; rehabilitation medicine.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: This study has received a contribution of epidural stimulation devices from St. Jude Medical/Abbott managed by the University of Minnesota. DPD has provisional patents for optimisation methods spinal cord stimulation and is also the CMO and owner of Stimsherpa Neuromodulation. US’s lab has received donations from Abbott through the J. Aron Allen Foundation. AK has received research grants from the Praxis Spinal Cord Institute through the University of British Columbia. He is also on the Coloplast and Convatech advisory boards and is the president of the American Spinal Cord Injury Association.
Figures
References
-
- Consortium for Spinal Cord Medicine . Acute management of autonomic dysreflexia: individuals with spinal cord injury presenting to health-care facilities. J Spinal Cord Med 2002;25 Suppl 1:S67–88. - PubMed
-
- Consortium for Spinal Cord Medicine . Neurogenic bowel management in adults with spinal cord injury. Paralyzed Veterans of America, 1998. Available: https://pva-cdnendpoint.azureedge.net/prod/libraries/media/pva/library/p...
-
- Consortium for Spinal Cord Medicine . Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals second edition. Paralyzed veterans of America, 2014. Available: https://pva-cdnendpoint.azureedge.net/prod/libraries/media/pva/library/p... - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
