Temporal trends and differences of SARS-CoV-2-specific antibody responses in symptomatic and asymptomatic subjects: a longitudinal study from Umbria in Italy
- PMID: 35851013
- PMCID: PMC9296997
- DOI: 10.1136/bmjopen-2021-056370
Temporal trends and differences of SARS-CoV-2-specific antibody responses in symptomatic and asymptomatic subjects: a longitudinal study from Umbria in Italy
Abstract
Objectives: Dynamics of antibody responses following SARS-CoV-2 infection are controversial in terms of immunity and persistence. We aimed to assess longitudinally the trend of antibody serological titres, their correlation with clinical severity as well as clinical reinfection during a follow-up.
Design: Longitudinal cohort, 12 months follow-up study.
Setting: USL Umbria 2.
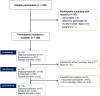
Participants: Consecutive subjects aged 15-75 who were discharged with the diagnosis of Sars-Cov-2 from the hospitals of the AUSL Umbria 2, or resulted positive to a PCR test for SARS-CoV-2 infection with or without symptoms were recruited. SARS-CoV-2 serological testing for antibodies targeting the Nucleocapside and Spike proteins were determined.
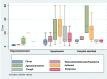
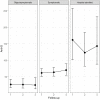
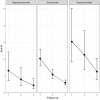
Results: Of 184 eligible subjects, 149 were available for evaluation: 17 were classified as oligo/asymptomatic, 107 as symptomatic, 25 as hospital admitted. Participants differed in terms of signs and symptoms as well as treatment. Overall there was a significant difference in terms of antibody titres between groups (anti-S: p<0.00; anti-N: p=0.019). Median anti-S titres in the symptomatic and hospital admitted participants were significantly higher compared with the oligo/asymptomatic participants. During follow-up, the median titre of anti-S antibodies did not show significant variations (p=0.500) and the difference within groups remained constant overtime. Subjects that showed an anti-S titre above the threshold of 12 U/mL were 88.7% at first visit and 88.2% at last follow-up. Anti-N values were higher in the hospital admitted participants compared with the other two groups. Anti-N titre reduced constantly overtime (p<0.001) and across the three groups of participants. The percentage of the subjects with serological titre above threshold (<1.4 U/mL) decreased from 74.5%% to 29.2% (p<0.001). None of the participants developed clinically evident reinfection.
Conclusion: Anti-N and anti-S correlate well with clinical severity. While anti-N declines overtime, anti-S antibodies persist for at least 1 year.
Keywords: COVID-19; immunology; infectious diseases; virology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Longitudinal humoral antibody response to SARS-CoV-2 infection among healthcare workers in a New York City hospital.BMJ Open. 2021 Oct 26;11(10):e051045. doi: 10.1136/bmjopen-2021-051045. BMJ Open. 2021. PMID: 34702729 Free PMC article.
-
Mild SARS-CoV-2 Illness Is Not Associated with Reinfections and Provides Persistent Spike, Nucleocapsid, and Virus-Neutralizing Antibodies.Microbiol Spectr. 2021 Oct 31;9(2):e0008721. doi: 10.1128/Spectrum.00087-21. Epub 2021 Sep 1. Microbiol Spectr. 2021. PMID: 34468184 Free PMC article.
-
Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: a longitudinal observational cohort study in western Greece.BMJ Open. 2022 May 19;12(5):e057084. doi: 10.1136/bmjopen-2021-057084. BMJ Open. 2022. PMID: 35589363 Free PMC article.
-
Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents.medRxiv [Preprint]. 2020 Aug 6:2020.08.05.20169128. doi: 10.1101/2020.08.05.20169128. medRxiv. 2020. Update in: J Infect Dis. 2021 Feb 13;223(3):389-398. doi: 10.1093/infdis/jiaa659. PMID: 32793928 Free PMC article. Updated. Preprint.
-
Low risk of reinfections and relation with serological response after recovery from the first wave of COVID-19.Eur J Clin Microbiol Infect Dis. 2021 Dec;40(12):2597-2604. doi: 10.1007/s10096-021-04335-x. Epub 2021 Aug 11. Eur J Clin Microbiol Infect Dis. 2021. PMID: 34378086 Free PMC article.
Cited by
-
Tracking the evolution of anti-SARS-CoV-2 antibodies and long-term humoral immunity within 2 years after COVID-19 infection.Sci Rep. 2024 Jun 11;14(1):13417. doi: 10.1038/s41598-024-64414-9. Sci Rep. 2024. PMID: 38862731 Free PMC article.
-
Rapid, early, and potent Spike-directed IgG, IgM, and IgA distinguish asymptomatic from mildly symptomatic COVID-19 in Uganda, with IgG persisting for 28 months.Front Immunol. 2023 Mar 16;14:1152522. doi: 10.3389/fimmu.2023.1152522. eCollection 2023. Front Immunol. 2023. PMID: 37006272 Free PMC article.
-
Clinical and laboratory considerations: determining an antibody-based composite correlate of risk for reinfection with SARS-CoV-2 or severe COVID-19.Front Public Health. 2023 Dec 28;11:1290402. doi: 10.3389/fpubh.2023.1290402. eCollection 2023. Front Public Health. 2023. PMID: 38222091 Free PMC article.
-
An Optimised Indirect ELISA Protocol for Detection and Quantification of Anti-viral Antibodies in Human Plasma or Serum: A Case Study Using SARS-CoV-2.Bio Protoc. 2023 Dec 20;13(24):e4905. doi: 10.21769/BioProtoc.4905. eCollection 2023 Dec 20. Bio Protoc. 2023. PMID: 38156036 Free PMC article.
-
Feasibility of self-organized blood sample collection in adults for study purposes in a primary care setting.PLoS One. 2023 May 25;18(5):e0286014. doi: 10.1371/journal.pone.0286014. eCollection 2023. PLoS One. 2023. PMID: 37228048 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
