Bone remodeling: an operational process ensuring survival and bone mechanical competence
- PMID: 35851054
- PMCID: PMC9293977
- DOI: 10.1038/s41413-022-00219-8
Bone remodeling: an operational process ensuring survival and bone mechanical competence
Abstract
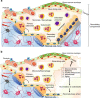
Bone remodeling replaces old and damaged bone with new bone through a sequence of cellular events occurring on the same surface without any change in bone shape. It was initially thought that the basic multicellular unit (BMU) responsible for bone remodeling consists of osteoclasts and osteoblasts functioning through a hierarchical sequence of events organized into distinct stages. However, recent discoveries have indicated that all bone cells participate in BMU formation by interacting both simultaneously and at different differentiation stages with their progenitors, other cells, and bone matrix constituents. Therefore, bone remodeling is currently considered a physiological outcome of continuous cellular operational processes optimized to confer a survival advantage. Bone remodeling defines the primary activities that BMUs need to perform to renew successfully bone structural units. Hence, this review summarizes the current understanding of bone remodeling and future research directions with the aim of providing a clinically relevant biological background with which to identify targets for therapeutic strategies in osteoporosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Frost, H. M. Bone remodeling dynamics (Charles C Thomas Company, 1963).
-
- Frost HM. A synchronous group of mammalian cells whose in vivo behavior can be studied. Henry Ford. Hosp. Med. Bull. 1965;13:161–172. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

