Facile Conversion of α-Amino Acids into α-Amino Phosphonates by Decarboxylative Phosphorylation using Visible-Light Photocatalysis
- PMID: 35851520
- PMCID: PMC9543399
- DOI: 10.1002/anie.202207063
Facile Conversion of α-Amino Acids into α-Amino Phosphonates by Decarboxylative Phosphorylation using Visible-Light Photocatalysis
Abstract
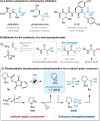
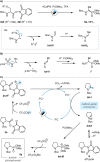
Amino phosphonates exhibit potent inhibitory activity for a wide range of biological processes due to their specific structural and electronic properties, making them important in a plethora of applications, including as enzyme inhibitors, herbicides, antiviral, antibacterial, and antifungal agents. While the traditional synthesis of α-amino phosphonates has relied on the multicomponent Kabachnik-Fields reaction, we herein describe a novel and facile conversion of activated derivatives of α-amino acids directly to their respective α-amino phosphonate counterparts via a decarboxylative radical-polar crossover process enabled by the use of visible-light organophotocatalysis. The novel method shows broad applicability across a range of natural and synthetic amino acids, operates under mild conditions, and has been demonstrated to successfully achieve the late-stage functionalization of drug molecules.
Keywords: Amino Acids; Phosphorylation; Photoredox Catalysis; Radical-Polar Crossover; Reaction Mechanisms.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Horiguchi M., Kandatstu M., Nature 1959, 184, 901–902. - PubMed
-
- Mastalerz P., Arch. Immunol. Ter. Dosw. 1959, 7, 201–210.
-
- Kafarski P., Lejczak B., Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193–215.
-
- Kukhar V. P. in Aminophosphonic and aminophosphinic acids: chemistry and biological activity, Wiley, New York, 2000.
-
- Mucha A., Kafarski P., Berlicki Ł., J. Med. Chem. 2011, 54, 5955–5980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

