Delivering the Promise of Gene Therapy with Nanomedicines in Treating Central Nervous System Diseases
- PMID: 35851766
- PMCID: PMC9475540
- DOI: 10.1002/advs.202201740
Delivering the Promise of Gene Therapy with Nanomedicines in Treating Central Nervous System Diseases
Abstract
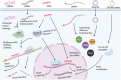
Central Nervous System (CNS) diseases, such as Alzheimer's diseases (AD), Parkinson's Diseases (PD), brain tumors, Huntington's disease (HD), and stroke, still remain difficult to treat by the conventional molecular drugs. In recent years, various gene therapies have come into the spotlight as versatile therapeutics providing the potential to prevent and treat these diseases. Despite the significant progress that has undoubtedly been achieved in terms of the design and modification of genetic modulators with desired potency and minimized unwanted immune responses, the efficient and safe in vivo delivery of gene therapies still poses major translational challenges. Various non-viral nanomedicines have been recently explored to circumvent this limitation. In this review, an overview of gene therapies for CNS diseases is provided and describes recent advances in the development of nanomedicines, including their unique characteristics, chemical modifications, bioconjugations, and the specific applications that those nanomedicines are harnessed to deliver gene therapies.
Keywords: bio-nanotechnology; blood-brain barrier; central nervous system diseases; gene therapy; nanomedicine.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Feigin V. L., Abajobir A. A., Abate K. H., Abd‐Allah F., Abdulle A. M., Abera S. F., Abyu G. Y., Ahmed M. B., Aichour A. N., Aichour I., Aichour M. T. E., Akinyemi R. O., Alabed S., AI‐Raddadi R., Alvis‐Guzman N., Zunt J. R., Murray C. J. L., Vos T., Lancet Neurol. 2017, 16, 877. - PubMed
-
- Feigin V. L., Nichols E., Alam T., Bannick M. S., Beghi E., Blake N., Culpepper W. J., Dorsey E. R., Elbaz A., Ellenbogen R. G., Fisher J. L., Fitzmaurice C., Giussani G., Glennie L., Murray C. J. L., Vos T., Lancet Neuro. 2019, 18, 459.
-
- World Health Organization , Neurological disorders: public health challenges, World Health Organization, Geneva 2006.
-
- Masters C. L., Bateman R., Blennow K., Rowe C. C., Sperling R. A., Cummings J. L., Nat. Rev. Dis. Primers 2015, 1, 15056. - PubMed
-
- Atri A., Semin. Neurol. 2019, 39, 227. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
