Solid-State Janus Nanoprecipitation Enables Amorphous-Like Heat Conduction in Crystalline Mg3 Sb2 -Based Thermoelectric Materials
- PMID: 35851767
- PMCID: PMC9443448
- DOI: 10.1002/advs.202202594
Solid-State Janus Nanoprecipitation Enables Amorphous-Like Heat Conduction in Crystalline Mg3 Sb2 -Based Thermoelectric Materials
Abstract
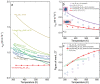
Solid-state precipitation can be used to tailor material properties, ranging from ferromagnets and catalysts to mechanical strengthening and energy storage. Thermoelectric properties can be modified by precipitation to enhance phonon scattering while retaining charge-carrier transmission. Here, unconventional Janus-type nanoprecipitates are uncovered in Mg3 Sb1.5 Bi0.5 formed by side-by-side Bi- and Ge-rich appendages, in contrast to separate nanoprecipitate formation. These Janus nanoprecipitates result from local comelting of Bi and Ge during sintering, enabling an amorphous-like lattice thermal conductivity. A precipitate size effect on phonon scattering is observed due to the balance between alloy-disorder and nanoprecipitate scattering. The thermoelectric figure-of-merit ZT reaches 0.6 near room temperature and 1.6 at 773 K. The Janus nanoprecipitation can be introduced into other materials and may act as a general property-tailoring mechanism.
Keywords: Janus nanoprecipitation; Mg3Sb2; atom probe tomography; low thermal conductivity; thermalelectrics.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Snyder G. J., Toberer E. S., Nat. Mater. 2008, 7, 105. - PubMed
-
- Jiang B., Yu Y., Cui J., Liu X., Xie L., Liao J., Zhang Q., Huang Y., Ning S., Jia B., Zhu B., Bai S., Chen L., Pennycook S. J., He J., Science 2021, 371, 830. - PubMed
-
- Kim W., Zide J., Gossard A., Klenov D., Stemmer S., Shakouri A., Majumdar A., Phys. Rev. Lett. 2006, 96, 045901. - PubMed
-
- Hsu K. F., Loo S., Guo F., Chen W., Dyck J. S., Uher C., Hogan T., Polychroniadis E. K., Kanatzidis M. G., Science 2004, 303, 818. - PubMed
Grants and funding
- 2018YFB0703600/National Key Research and Development Program of China
- 51872133/National Natural Science Foundation of China
- 2021B1212040001/Guangdong Provincial Key Laboratory Program
- Department of Science and Technology of Guangdong Province
- 2009 00971/Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University
- KAW-2020.0196/Knut and Alice Wallenberg foundation through the Wallenberg Academy Fellows program
- Linköping Electron Microscopy Laboratory
- RIF 14-0074/Research Infrastructure Fellow
- SFB 917/German Science Foundation
- SFB 917/Deutsche Forschungsgemeinschaft
- RIF14-0074/Stiftelsen för Strategisk Forskning
- Stiftelsen för Strategisk Forskning
- 0074/Stiftelsen för Strategisk Forskning
- support for the Linköping Electron Microscopy Laboratory/Knut och Alice Wallenbergs Stiftelse
- XPLORER PRIZE/Tencent
- 2018YFB0703600/Tencent
LinkOut - more resources
Full Text Sources
