Sustained high body temperature exacerbates cognitive function and Alzheimer's disease-related pathologies
- PMID: 35851831
- PMCID: PMC9293958
- DOI: 10.1038/s41598-022-16626-0
Sustained high body temperature exacerbates cognitive function and Alzheimer's disease-related pathologies
Abstract
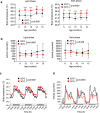
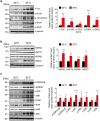
Global warming is a serious public health threat to people worldwide. High body temperature is one of the important risk factors for Alzheimer's disease (AD), and the body temperature of AD patients has been found to be significantly higher than that of elderly control subjects. However, the effects of high body temperature on cognitive function and AD pathologies have not been completely elucidated. We report here that Tg2576 mice housed at a high ambient temperature of 30 °C for 13 months showed an increase in the body temperature, which is accompanied by memory impairment and an enhancement of amyloid-β peptides (Aβ) generation through the upregulation of β-site APP cleaving enzyme 1 (BACE1) level and decrease in the level of an Aβ-degrading enzyme, neprilysin (NEP) in the brain, compared with those of Tg2576 mice at 23 °C. High body temperature also increased the levels of heat shock proteins (HSPs), stress-stimulated kinases such as JNK, and total tau, leading to the enhancement of tau phosphorylation at 30 °C. Taken together, our findings suggest that high body temperature exacerbates cognitive function and AD pathologies, which provides a mechanistic insight for its prevention.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Deletion of UCP1 in Tg2576 Mice Increases Body Temperature and Exacerbates Alzheimer's Disease-Related Pathologies.Int J Mol Sci. 2023 Feb 1;24(3):2741. doi: 10.3390/ijms24032741. Int J Mol Sci. 2023. PMID: 36769062 Free PMC article.
-
A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice.Mol Brain. 2015 Mar 25;8:19. doi: 10.1186/s13041-015-0110-5. Mol Brain. 2015. PMID: 25884928 Free PMC article.
-
Relationship between ubiquilin-1 and BACE1 in human Alzheimer's disease and APdE9 transgenic mouse brain and cell-based models.Neurobiol Dis. 2016 Jan;85:187-205. doi: 10.1016/j.nbd.2015.11.005. Epub 2015 Nov 10. Neurobiol Dis. 2016. PMID: 26563932
-
The β-Secretase Enzyme BACE1: A Biochemical Enigma for Alzheimer's Disease.CNS Neurol Disord Drug Targets. 2020;19(3):184-194. doi: 10.2174/1871527319666200526144141. CNS Neurol Disord Drug Targets. 2020. PMID: 32452328 Review.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
-
Dementia and Risks of Temperature-Related Mortality and Hospitalizations in Germany.J Gerontol A Biol Sci Med Sci. 2025 Mar 7;80(4):glae292. doi: 10.1093/gerona/glae292. J Gerontol A Biol Sci Med Sci. 2025. PMID: 39660614 Free PMC article.
-
Co-administration of Nanowired Oxiracetam and Neprilysin with Monoclonal Antibodies to Amyloid Beta Peptide and p-Tau Thwarted Exacerbation of Brain Pathology in Concussive Head Injury at Hot Environment.Adv Neurobiol. 2023;32:271-313. doi: 10.1007/978-3-031-32997-5_7. Adv Neurobiol. 2023. PMID: 37480464 Review.
-
Extreme Heat and Hospitalization Among Older Persons With Alzheimer Disease and Related Dementias.JAMA Intern Med. 2025 Apr 1;185(4):412-421. doi: 10.1001/jamainternmed.2024.7719. JAMA Intern Med. 2025. PMID: 39899291
-
Thermotherapy has Sexually Dimorphic Responses in APP/PS1 Mice.bioRxiv [Preprint]. 2024 Aug 8:2024.03.26.586836. doi: 10.1101/2024.03.26.586836. bioRxiv. 2024. Update in: Aging (Albany NY). 2024 Nov 29;16(21):13237-13251. doi: 10.18632/aging.206156. PMID: 38586039 Free PMC article. Updated. Preprint.
-
Deletion of UCP1 in Tg2576 Mice Increases Body Temperature and Exacerbates Alzheimer's Disease-Related Pathologies.Int J Mol Sci. 2023 Feb 1;24(3):2741. doi: 10.3390/ijms24032741. Int J Mol Sci. 2023. PMID: 36769062 Free PMC article.
References
-
- Bufill E, et al. Genetic and environmental factors that may influence in the senile form of Alzheimer's disease: Nested case control studies. Neurologia. 2009;24:108–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

