Drop-of-sample rheometry of biological fluids by noncontact acoustic tweezing spectroscopy
- PMID: 35851909
- PMCID: PMC10661770
- DOI: 10.1039/d2lc00356b
Drop-of-sample rheometry of biological fluids by noncontact acoustic tweezing spectroscopy
Abstract
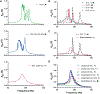
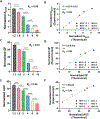
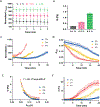
Knowledge of rheological properties, such as viscosity and elasticity, is necessary for efficient material processing and transportation as well as biological analysis. Existing rheometers operate with large sample volume and induce sample contact with container or device walls, which are inadequate for rheological analysis of sensitive fluids limited in availability. In this work, we introduce acoustic tweezing spectroscopy (ATS), a novel noncontact rheological technique that operates with a single 4-6 μl drop of fluid sample. In ATS, a sample drop is acoustically levitated and then exposed to a modulated acoustic signal to induce its forced oscillation. The time-dependent sample viscosity and elasticity are measured from the resulting drop response. The ATS measurements of polymeric solutions (dextran, xanthan gum, gelatin) agree well with previously reported data. The ATS predicts that the shear viscosity of blood plasma increases from 1.5 cP at 1.5 min of coagulation onset to 3.35 cP at 9 min, while its shear elastic modulus grows from a negligible value to 10.7 Pa between 3.5 min and 6.5 min. Coagulation increases whole blood viscosity from 5.4 cP to 20.7 cP and elasticity from 0.1 Pa to 19.2 Pa at 15 min. In summary, ATS provides the opportunity for sensitive small-volume rheological analysis in biomedical research and medical, pharmaceutical, and chemical industries.
Conflict of interest statement
Conflicts of interest
N. K. and D. B. K. have the following conflicts of interest to disclose: N. K. owns equity and has an employment at Levisonics Inc. She is also an inventor listed on the patent PCT/US21/15336 (pending). D. B. K. owns equity in Levisonics Inc. He is also an inventor on patents PCT/US14/55559 (issued), PCT/US2018/014879 (issued), and PCT/US21/15336 (pending). J. C. O. declares no conflict of interest.
Figures


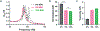
References
-
- Borzacchiello A, Ambrosio L, Netti P and Nicolais L, in Tissue Engineering and Novel Delivery Systems, CRC Press, 2003, pp. 341–361.
-
- Dealy JM and Wissbrun KF, in Melt Rheology and Its Role in Plastics Processing, Springer, 1999, pp. 567–600.
-
- Tabilo-Munizaga G and Barbosa-Cánovas GV, J. Food Eng, 2005, 67, 147–156.
-
- Gómez-Díaz D and Navaza JM, J. Food Eng, 2003, 56, 387–392.
-
- Ilyin SO and Strelets LA, Energy Fuels, 2018, 32, 268–278.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

