Sphingomyelinase activity promotes atrophy and attenuates force in human muscle fibres and is elevated in heart failure patients
- PMID: 35852046
- PMCID: PMC9530516
- DOI: 10.1002/jcsm.13029
Sphingomyelinase activity promotes atrophy and attenuates force in human muscle fibres and is elevated in heart failure patients
Abstract
Background: Activation of sphingomyelinase (SMase) as a result of a general inflammatory response has been implicated as a mechanism underlying disease-related loss of skeletal muscle mass and function in several clinical conditions including heart failure. Here, for the first time, we characterize the effects of SMase activity on human muscle fibre contractile function and assess skeletal muscle SMase activity in heart failure patients.
Methods: The effects of SMase on force production and intracellular Ca2+ handling were investigated in single intact human muscle fibres. Additional mechanistic studies were performed in single mouse toe muscle fibres. RNA sequencing was performed in human muscle bundles exposed to SMase. Intramuscular SMase activity was measured from heart failure patients (n = 61, age 69 ± 0.8 years, NYHA III-IV, ejection fraction 25 ± 1.0%, peak VO2 14.4 ± 0.6 mL × kg × min) and healthy age-matched control subjects (n = 10, age 71 ± 2.2 years, ejection fraction 60 ± 1.2%, peak VO2 25.8 ± 1.1 mL × kg × min). SMase activity was related to circulatory factors known to be associated with progression and disease severity in heart failure.
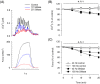
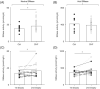
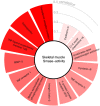
Results: Sphingomyelinase reduced muscle fibre force production (-30%, P < 0.05) by impairing sarcoplasmic reticulum (SR) Ca2+ release (P < 0.05) and reducing myofibrillar Ca2+ sensitivity. In human muscle bundles exposed to SMase, RNA sequencing analysis revealed 180 and 291 genes as up-regulated and down-regulated, respectively, at a FDR of 1%. Gene-set enrichment analysis identified 'proteasome degradation' as an up-regulated pathway (average fold-change 1.1, P = 0.008), while the pathway 'cytoplasmic ribosomal proteins' (average fold-change 0.8, P < 0.0001) and factors involving proliferation of muscle cells (average fold-change 0.8, P = 0.0002) where identified as down-regulated. Intramuscular SMase activity was ~20% higher (P < 0.05) in human heart failure patients than in age-matched healthy controls and was positively correlated with markers of disease severity and progression, and with several circulating inflammatory proteins, including TNF-receptor 1 and 2. In a longitudinal cohort of heart failure patients (n = 6, mean follow-up time 2.5 ± 0.2 years), SMase activity was demonstrated to increase by 30% (P < 0.05) with duration of disease.
Conclusions: The present findings implicate activation of skeletal muscle SMase as a mechanism underlying human heart failure-related loss of muscle mass and function. Moreover, our findings strengthen the idea that SMase activation may underpin disease-related loss of muscle mass and function in other clinical conditions, acting as a common patophysiological mechanism for the myopathy often reported in diseases associated with a systemic inflammatory response.
Keywords: Ca2+ sensitivity RNAseq; Heart failure; Skeletal muscle; Sphingomyelinas.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors have no competing interests.
Figures


Similar articles
-
Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers.J Appl Physiol (1985). 2012 May;112(9):1538-45. doi: 10.1152/japplphysiol.01269.2011. Epub 2012 Feb 23. J Appl Physiol (1985). 2012. PMID: 22362402 Free PMC article.
-
Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow.Eur Heart J. 2007 Apr;28(7):821-8. doi: 10.1093/eurheartj/ehl541. Epub 2007 Mar 12. Eur Heart J. 2007. PMID: 17353227
-
Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue.Am J Physiol Cell Physiol. 2010 Sep;299(3):C552-60. doi: 10.1152/ajpcell.00065.2010. Epub 2010 Jun 2. Am J Physiol Cell Physiol. 2010. PMID: 20519448 Free PMC article.
-
Neutral sphingomyelinase: past, present and future.Chem Phys Lipids. 1999 Nov;102(1-2):79-96. doi: 10.1016/s0009-3084(99)00077-8. Chem Phys Lipids. 1999. PMID: 11001563 Review.
-
Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor.J Leukoc Biol. 1998 Jun;63(6):678-82. doi: 10.1002/jlb.63.6.678. J Leukoc Biol. 1998. PMID: 9620659 Review.
Cited by
-
25-Hydroxycholesterol modulates synaptic vesicle endocytosis at the mouse neuromuscular junction.Pflugers Arch. 2025 Mar;477(3):421-439. doi: 10.1007/s00424-024-03058-0. Epub 2025 Jan 9. Pflugers Arch. 2025. PMID: 39786596
-
Sarcopenia and cardiovascular diseases: A systematic review and meta-analysis.J Cachexia Sarcopenia Muscle. 2023 Jun;14(3):1183-1198. doi: 10.1002/jcsm.13221. Epub 2023 Apr 1. J Cachexia Sarcopenia Muscle. 2023. PMID: 37002802 Free PMC article.
-
Sphingolipid Metabolism and Signalling Pathways in Heart Failure: From Molecular Mechanism to Therapeutic Potential.J Inflamm Res. 2025 Apr 23;18:5477-5498. doi: 10.2147/JIR.S515757. eCollection 2025. J Inflamm Res. 2025. PMID: 40291458 Free PMC article. Review.
-
β2-Adrenergic Regulation of the Neuromuscular Transmission and Its Lipid-Dependent Switch.Mol Neurobiol. 2024 Sep;61(9):6805-6821. doi: 10.1007/s12035-024-03991-2. Epub 2024 Feb 14. Mol Neurobiol. 2024. PMID: 38353924
-
Mechanism of Purinergic Regulation of Neurotransmission in Mouse Neuromuscular Junction: The Role of Redox Signaling and Lipid Rafts.Neurochem Res. 2024 Aug;49(8):2021-2037. doi: 10.1007/s11064-024-04153-5. Epub 2024 May 30. Neurochem Res. 2024. PMID: 38814360
References
-
- Doehner W, Bunck AC, Rauchhaus M, von Haehling S, Brunkhorst FM, Cicoira M. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J 2007;28:821–828. - PubMed
-
- Mebarek S, Komati H, Naro F, Zeiller C, Alvisi M, Lagarde M. Inhibition of de novo ceramide synthesis upregulates phospholipase D and enhances myogenic differentiation. J Cell Sci 2007;120:407–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

