Cardiac events among patients with sarcoma treated with doxorubicin by method of infusion: A real-world database study
- PMID: 35852051
- PMCID: PMC9875654
- DOI: 10.1002/cnr2.1681
Cardiac events among patients with sarcoma treated with doxorubicin by method of infusion: A real-world database study
Abstract
Background: Administration of doxorubicin by continuous intravenous (CIV) infusion, versus bolus (BOL) administration, has been proposed to mitigate the risk of cardiac events. This study used real-world data to explore the association between mode of doxorubicin administration and duration of treatment, time-to-treatment failure (TTF), and cardiac events.
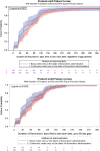
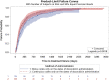
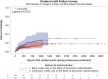
Methods: Occurrence of cardiac events after initiation of BOL versus CIV doxorubicin for sarcoma in the International Business Machines MarketScan claims database were compared. Duration of doxorubicin treatment, TTF, and time-to-first-cardiac event (TCE) were evaluated using Kaplan-Meier method and unadjusted and adjusted Cox regression models.
Results: A total of 196 patients were included in the BOL group and 399 in the CIV group. In unadjusted analyses, there were significant differences between BOL versus CIV for duration of doxorubicin treatment (median 1.4 vs. 2.1 months, p = .002), TTF (median 8.8 vs. 5.6 months, p = .002), and TCE (medians not reached, p = .03). Adjusting for baseline covariates, only TTF remained significant (hazard ratio: 0.71, 95% confidence interval 0.59-0.86, p = .0004), favoring BOL.
Conclusions: While the risk of cardiac complications was higher with BOL in unadjusted analysis, the risk was no longer present in the adjusted analysis. While we cannot draw causal inferences due to the retrospective, nonrandomized study design, these data suggest that replacing BOL with prolonged CIV administration has not been effective as a strategy to mitigate cardiac events, given community standards of oncologic practice.
Keywords: anthracycline; bolus; cardiotoxicity; doxorubicin; infusion; retrospective; sarcoma.
© 2022 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Bolus versus Continuous Intravenous Delivery of Doxorubicin in Soft-Tissue Sarcomas: Post Hoc Analysis of a Prospective Randomized Trial (SARC021/TH CR-406).Clin Cancer Res. 2023 Mar 14;29(6):1068-1076. doi: 10.1158/1078-0432.CCR-22-1564. Clin Cancer Res. 2023. PMID: 36622694 Clinical Trial.
-
A prospective randomized trial of adjuvant chemotherapy with bolus versus continuous infusion of doxorubicin in patients with high-grade extremity soft tissue sarcoma and an analysis of prognostic factors.Cancer. 1991 Sep 15;68(6):1221-9. doi: 10.1002/1097-0142(19910915)68:6<1221::aid-cncr2820680607>3.0.co;2-r. Cancer. 1991. PMID: 1873773 Clinical Trial.
-
Safety and efficacy of the multidrug-resistance inhibitor biricodar (VX-710) with concurrent doxorubicin in patients with anthracycline-resistant advanced soft tissue sarcoma.Clin Cancer Res. 2002 Feb;8(2):383-93. Clin Cancer Res. 2002. PMID: 11839653 Clinical Trial.
-
Comparison of bolus administration and short-term infusion versus long-term infusion of doxorubicin in terms of cardiotoxicity and efficacy.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jun;397(6):3771-3780. doi: 10.1007/s00210-023-02886-8. Epub 2023 Dec 14. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38095650 Review.
-
Chemotherapy of advanced sarcomas of bone and soft tissue.Semin Oncol. 1992 Dec;19(6 Suppl 12):13-20. Semin Oncol. 1992. PMID: 1485169 Review.
Cited by
-
The Rate of Infusion Represents an Important Aspect of Administering Anticancer Agents.Risk Manag Healthc Policy. 2023 Nov 22;16:2531-2541. doi: 10.2147/RMHP.S442692. eCollection 2023. Risk Manag Healthc Policy. 2023. PMID: 38024501 Free PMC article. Review.
-
In vivo and in situ monitoring of doxorubicin pharmacokinetics with an implantable bioresorbable optical sensor.Sci Adv. 2025 Apr 18;11(16):eads0265. doi: 10.1126/sciadv.ads0265. Epub 2025 Apr 16. Sci Adv. 2025. PMID: 40238874 Free PMC article.
References
-
- Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first‐line treatment of advanced or metastatic soft‐tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415‐423. - PubMed
-
- Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: a phase III, placebo‐controlled study of doxorubicin with or without Palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;34(32):3898‐3905. - PubMed
-
- Tap WD, Papai Z, van Tine BA, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft‐tissue sarcoma (TH CR‐406/SARC021): an international, multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2017;18(8):1089‐1103. - PMC - PubMed
-
- von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin‐lnduced congestive heart failure. Ann Intern Med. 1979;91(5):710‐717. - PubMed
-
- Minow RA, Benjamin RS, Lee ET, Gottlieb JA. Adriamycin cardiomyopathy—risk factors. Cancer. 1977;39(4):1397‐1402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

