Novel types of frequency filtering in the lateral perforant path projections to dentate gyrus
- PMID: 35852108
- PMCID: PMC9513824
- DOI: 10.1113/JP283012
Novel types of frequency filtering in the lateral perforant path projections to dentate gyrus
Abstract
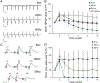
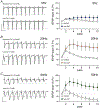
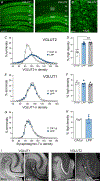
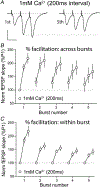
Despite its evident importance to learning theory and models, the manner in which the lateral perforant path (LPP) transforms signals from entorhinal cortex to hippocampus is not well understood. The present studies measured synaptic responses in the dentate gyrus (DG) of adult mouse hippocampal slices during different patterns of LPP stimulation. Theta (5 Hz) stimulation produced a modest within-train facilitation that was markedly enhanced at the level of DG output. Gamma (50 Hz) activation resulted in a singular pattern with initial synaptic facilitation being followed by a progressively greater depression. DG output was absent after only two pulses. Reducing release probability with low extracellular calcium instated frequency facilitation to gamma stimulation while long-term potentiation, which increases release by LPP terminals, enhanced within-train depression. Relatedly, per terminal concentrations of VGLUT2, a vesicular glutamate transporter associated with high release probability, were much greater in the LPP than in CA3-CA1 connections. Attempts to circumvent the potent gamma filter using a series of short (three-pulse) 50 Hz trains spaced by 200 ms were only partially successful: composite responses were substantially reduced after the first burst, an effect opposite to that recorded in field CA1. The interaction between bursts was surprisingly persistent (>1.0 s). Low calcium improved throughput during theta/gamma activation but buffering of postsynaptic calcium did not. In all, presynaptic specializations relating to release probability produce an unusual but potent type of frequency filtering in the LPP. Patterned burst input engages a different type of filter with substrates that are also likely to be located presynaptically. KEY POINTS: The lateral perforant path (LPP)-dentate gyrus (DG) synapse operates as a low-pass filter, where responses to a train of 50 Hz, γ frequency activation are greatly suppressed. Activation with brief bursts of γ frequency information engages a secondary filter that persists for prolonged periods (lasting seconds). Both forms of LPP frequency filtering are influenced by presynaptic, as opposed to postsynaptic, processes; this contrasts with other hippocampal synapses. LPP frequency filtering is modified by the unique presynaptic long-term potentiation at this synapse. Computational simulations indicate that presynaptic factors associated with release probability and vesicle recycling may underlie the potent LPP-DG frequency filtering.
Keywords: VGLUT2; frequency facilitation; gamma oscillations; hippocampus; lateral entorhinal cortex; long-term potentiation; simulations; transmitter release.
© 2022 The Authors. The Journal of Physiology © 2022 The Physiological Society.
Figures









References
-
- Adelman JP, Maylie J, & Sah P. (2012). Small-conductance Ca2+-activated K+ channels: Form and function. Annual Review of Physiology, 74(1), 245–269. - PubMed
-
- Arai A, Silberg J, & Lynch G. (1995). Differences in the refractory properties of two distinct inhibitory circuitries in field CA1 of the hippocampus. Brain Research, 704(2), 298–306. - PubMed
-
- Barmashenko G, Hefft S, Aertsen A, Kirschstein T, & Kohling R. (2011). Positive shifts of the GABAA receptor reversal potential due to altered chloride homeostasis is widespread after status epilepticus. Epilepsia, 52(9), 1570–1578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

